The mineralogy of Berkelium
| About Berkelium |
|---|
| Berkelium is a radioactive transuranic element that has only been produced in nuclear reactors. It is possible that some berkelium and other transuranic elements were created in the natural nuclear reactor in Oklo, Gabon. |
| General Properties | |
|---|---|
| Symbol: | Bk |
| Atomic Number: | 97 |
| Standard atomic weight (Ar): | [247] |
| Electron configuration: | [Rn] 5f9 7s2 |
| Photos | ||
|---|---|---|
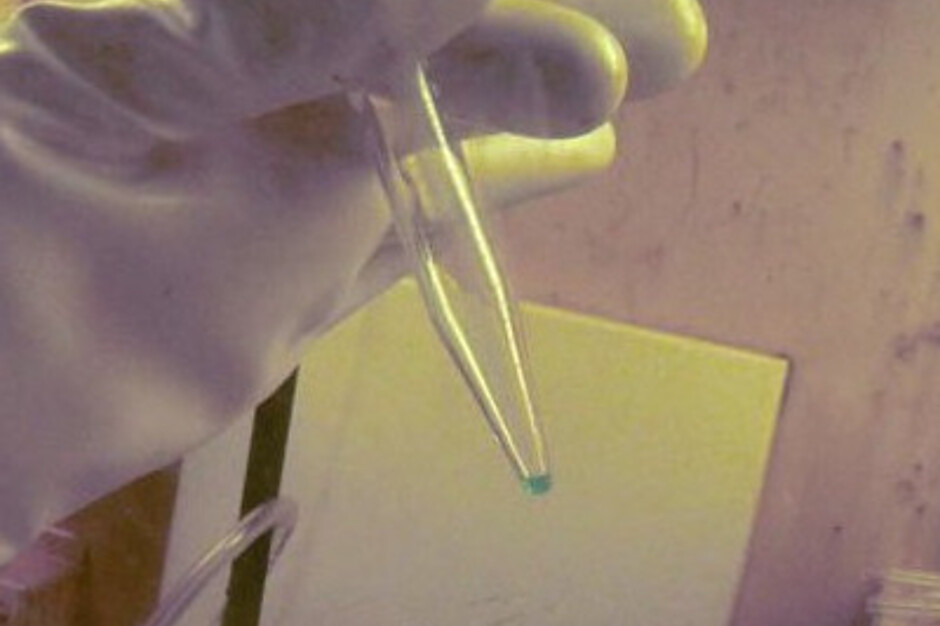
| < | 13 milligrams of berkelium 249 | > |
| Atomic Properties | |
|---|---|
| Electronegativity (Pauling scale): | 1.3 |
| Atomic Radius: | 170 pm |
| Ionic Radius: | 96 pm (+3) |
| 1st Ionization energy: | 601 kJ/mol |
| Oxidation States: | 3,4 |
| Physical Properties | |
|---|---|
| Standard State: | solid |
| Bonding Type: | metallic |
| Melting Point: | 1323 K |
| Density: | 14.78 g/cm3 |
| Metal/Non-Metal: | actinoid |
| Main isotopes of Berkelium | ||||
|---|---|---|---|---|
| Isotope | % in Nature | Half Life | Decay type | Decay product |
| 245Bk | synthetic | 4.94d | ε | 245Cm |
| α | 241Am | |||
| 246Bk | synthetic | 1.8d | α | 242Am |
| ε | 246Cm | |||
| 247Bk | synthetic | 1380y | α | 243Am |
| 248Bk | synthetic | >300y | α | 244Am |
| 249Bk | synthetic | 330d | α | 245Am |
| Spontaneous fission | ||||
| β− | 249Cf | |||
| Other Information | |
|---|---|
| Year Discovered: | 1949 |
| Discovered By: | Lawrence Berkeley National Laboratory |
| Named For: | after Berkeley, California, where it was discovered |
| CPK color coding: | #8A4FE3 |
| External Links: | WikipediaWebElementsLos Alamos National LaboratoryTheodore Gray's PeriodicTable.com |