The mineralogy of Argon
| About Argon |
|---|
| Argon is a noble gas and as such does not form any natural minerals. |
| General Properties | |
|---|---|
| Symbol: | Ar |
| Atomic Number: | 18 |
| Standard atomic weight (Ar): | 39.948(1) |
| Electron configuration: | [Ne] 3s2 3p6 |
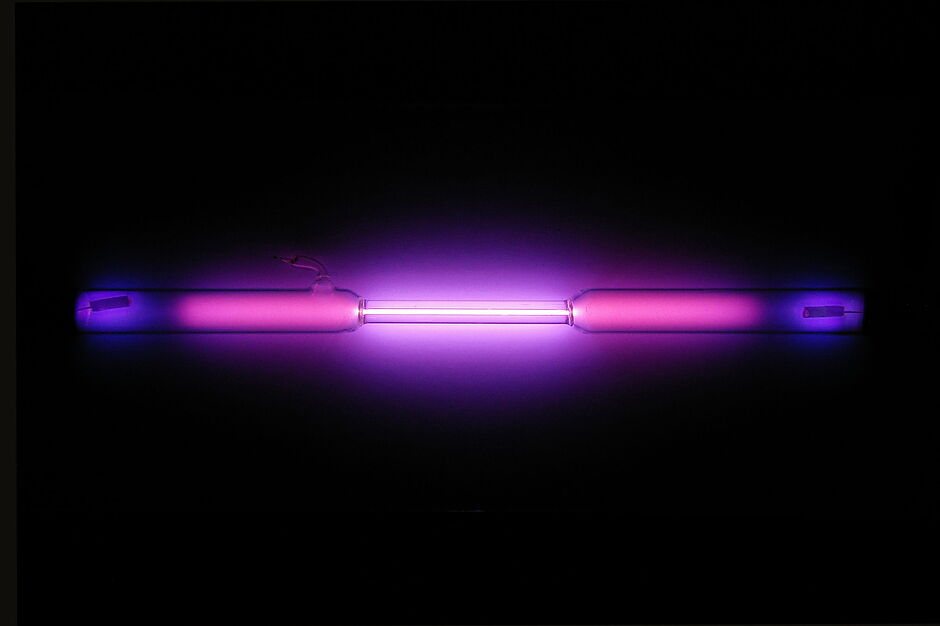
| Photos | ||
|---|---|---|
| < | Vial of glowing ultrapure argon | > |
| Atomic Properties | |
|---|---|
| Atomic Radius: | 71 pm |
| Van der Waals Radius: | 188 pm |
| 1st Ionization energy: | 1521 kJ/mol |
| Physical Properties | |
|---|---|
| Standard State: | gas |
| Bonding Type: | atomic |
| Melting Point: | 84 K |
| Boiling Point: | 87 K |
| Metal/Non-Metal: | noble gas |
| Main isotopes of Argon | ||||
|---|---|---|---|---|
| Isotope | % in Nature | Half Life | Decay type | Decay product |
| 36Ar | 0.337% | - | β+β+ ? | 36S |
| 37Ar | synthetic | 35d | ε | 37Cl |
| 38Ar | 0.063% | stable | ||
| 39Ar | trace | 269y | β− | 39K |
| 40Ar | 99.600% | stable | ||
| 41Ar | synthetic | 109.34min | β− | 41K |
| 42Ar | synthetic | 32.9y | β− | 42K |
| Other Information | |
|---|---|
| Year Discovered: | 1894 |
| Discovered By: | Lord Rayleigh, William Ramsay |
| Year Isolated: | 1894 |
| Isolated By: | Lord Rayleigh, William Ramsay |
| Named For: | from Greek meaning "inactive" |
| CPK color coding: | #80D1E3 |
| External Links: | WikipediaWebElementsLos Alamos National LaboratoryTheodore Gray's PeriodicTable.com |
| Geochemistry of Argon | |
|---|---|
| Goldschmidt classification: | Atmophile |
| Elemental Abundance for Argon | ||
|---|---|---|
| Crust (CRC Handbook) | 3.5 x 10-6 | mass fraction, kg/kg |
| Sea Water (CRC Handbook) | 4.5 x 10-7 | mass per volume fraction, kg/L |
| Sea Water (Kaye & Laby) | 4.5 x 10-7 | mass per volume fraction, kg/L |
| Atmosphere (NASA) | 9.340% | as Ar |
| The Sun (Kaye & Laby) | 1.0 x 10-1 | atom mole fraction relative to Si=1 |
| Solar System (Kaye & Laby) | 1.0 x 10-1 | atom mole fraction relative to Si=1 |
| Solar System (Ahrens) | 1.01 x 10-1 (6%) | atom mole fraction relative to Si=1 (% uncertainty) |
| Periodic Table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chlorine << Argon >> Potassium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spotted a mistake/omission? - These pages are a work in progress, so please send all comments/corrections to jolyon@mindat.org. Thank you.
Constants and physical property data from:
Lide, David R. - Ed. (2005) CRC Handbook of Chemistry and Physics - A Ready-Reference Book of Chemical and Physical Data (85th ed.). CRC Press.
National Physical Laboratory (2005) Kaye and Laby Online (discontinued). https://web.archive.org/web/20190506031327/http://www.kayelaby.npl.co.uk/
Kaye, G. W. C.; Laby, T. H. (n.d.) Tables of Physical and Chemical Constants and some Mathematical Functions. Longmans, Green, and Co.
Greenwood, N.N.; Earnshaw, A. (1997) Chemistry of the Elements (2nd ed.). Butterworth–Heinemann.
Ahrens, Thomas J. - Ed. (1995) Global Earth Physics - A Handbook of Physical Constants - AGU Reference Shelf No. 1. American Geophysical Union.
Railsback, L. Bruce (2003) An earth scientist's periodic table of the elements and their ions. Geology, 31 (9) 737 doi:10.1130/g19542.1
Emsley, John (2001) Nature's Building Blocks - An A-Z Guide to the Elements. Oxford University Press, Oxford.