Hexahydrite
A valid IMA mineral species - grandfathered
This page is currently not sponsored. Click here to sponsor this page.
About Hexahydrite
Formula:
MgSO4 · 6H2O
Colour:
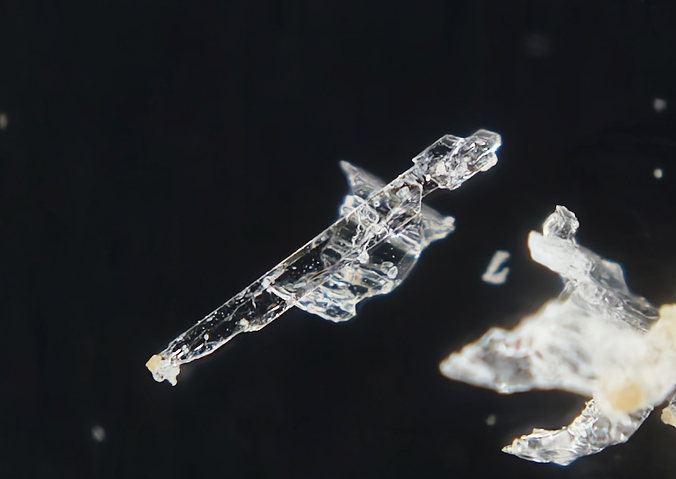
Colourless, white, pale greenish white; colourless in transmitted light.
Lustre:
Sub-Vitreous, Pearly, Dull, Earthy
Hardness:
2 - 2½
Specific Gravity:
1.757
Crystal System:
Monoclinic
Member of:
Name:
Named in 1911 by Robert Angus Alister Johnston in allusion to its composition, having six (HEXA-) molecules of water (HYDR-) in its formula.
Hexahydrite Group.
May dehydrate to starkeyite (Chou, 2005).
A supposed polymorph reported by Maynard-Casely et al. (2021) was shown by Fortes and Meusburger (2022) to have an incorrect formula.
May dehydrate to starkeyite (Chou, 2005).
A supposed polymorph reported by Maynard-Casely et al. (2021) was shown by Fortes and Meusburger (2022) to have an incorrect formula.
Unique Identifiers
Mindat ID:
1891
Long-form identifier:
mindat:1:1:1891:9
IMA Classification of Hexahydrite
Approved, 'Grandfathered' (first described prior to 1959)
IMA Formula:
Mg(SO4) · 6H2O
First published:
1911
Classification of Hexahydrite
7.CB.25
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
C : Sulfates (selenates, etc.) without additional anions, with H2O
B : With only medium-sized cations
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
C : Sulfates (selenates, etc.) without additional anions, with H2O
B : With only medium-sized cations
29.6.8.1
29 : HYDRATED ACID AND NORMAL SULFATES
6 : AXO4·xH2O
29 : HYDRATED ACID AND NORMAL SULFATES
6 : AXO4·xH2O
25.3.5
25 : Sulphates
3 : Sulphates of Mg
25 : Sulphates
3 : Sulphates of Mg
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
| Symbol | Source | Reference |
|---|---|---|
| Hhy | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
Pronunciation of Hexahydrite
Pronunciation:
| Play | Recorded by | Country |
|---|---|---|
| Jolyon Ralph | United Kingdom |
Physical Properties of Hexahydrite
Sub-Vitreous, Pearly, Dull, Earthy
Transparency:
Transparent, Translucent
Colour:
Colourless, white, pale greenish white; colourless in transmitted light.
Streak:
White
Hardness:
2 - 2½ on Mohs scale
Tenacity:
Brittle
Cleavage:
Perfect
On {100}, perfect.
On {100}, perfect.
Fracture:
Conchoidal
Density:
1.757 g/cm3 (Measured) 1.745 g/cm3 (Calculated)
Optical Data of Hexahydrite
Type:
Biaxial (-)
RI values:
nα = 1.426 nβ = 1.453 nγ = 1.456
2V:
Measured: 38° , Calculated: 36°
Max. Birefringence:
δ = 0.030
Based on recorded range of RI values above.
Based on recorded range of RI values above.
Interference Colours:
The colours simulate birefringence patterns seen in thin section under crossed polars. They do not take into account mineral colouration or opacity.
Michel-Levy Bar The default colours simulate the birefringence range for a 30 µm thin-section thickness. Adjust the slider to simulate a different thickness.
Grain Simulation You can rotate the grain simulation to show how this range might look as you rotated a sample under crossed polars.
The colours simulate birefringence patterns seen in thin section under crossed polars. They do not take into account mineral colouration or opacity.
Michel-Levy Bar The default colours simulate the birefringence range for a 30 µm thin-section thickness. Adjust the slider to simulate a different thickness.
Grain Simulation You can rotate the grain simulation to show how this range might look as you rotated a sample under crossed polars.
Surface Relief:
Moderate
Dispersion:
none
Optical Extinction:
Y = b; X ∧ c = 25°.
Chemistry of Hexahydrite
Mindat Formula:
MgSO4 · 6H2O
Element Weights:
Elements listed:
Crystallography of Hexahydrite
Crystal System:
Monoclinic
Class (H-M):
2/m - Prismatic
Space Group:
P2/m
Cell Parameters:
a = 24.44 Å, b = 7.21 Å, c = 10.11 Å
β = 98.28°
β = 98.28°
Ratio:
a:b:c = 3.39 : 1 : 1.402
Unit Cell V:
1,762.94 ų (Calculated from Unit Cell)
Morphology:
Good crystals rare; sometimes thick tabular {001}. Also coarse columnar to delicately fibrous to acicular.
Twinning:
1. On {001}; 2. on {110}.
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0009280 | Hexahydrite | Zalkin A, Ruben H, Templeton D H (1964) The crystal structure and hydrogen bonding of magnesium sulfate hexahydrate Acta Crystallographica 17 235-240 |  | 1964 | synthetic | 0 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Loading XRD data...
Data courtesy of RRUFF project at University of Arizona, used with permission.
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 5.45 Å | (50) |
| 5.10 Å | (45) |
| 4.88 Å | (30) |
| 4.39 Å | (100) |
| 4.16 Å | (35) |
| 4.04 Å | (45) |
| 2.94 Å | (30) |
Comments:
ICDD 24-719
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 2: Planetesimal differentiation and alteration | 4.566-4.550 |
| 6 : Secondary asteroid phases | 4.566-4.560 |
| Near-surface Processes | |
| 21 : Chemically precipitated carbonate, phosphate, iron formations | |
| 25 : Evaporites (prebiotic) | |
| Stage 7: Great Oxidation Event | <2.4 |
| 45b : [Other oxidized fumarolic minerals] | |
| Stage 10a: Neoproterozoic oxygenation/terrestrial biosphere | <0.6 |
| 49 : Oxic cellular biomineralization (see also #44) | <0.54 |
| 50 : Coal and/or oil shale minerals | <0.36 |
| Stage 10b: Anthropogenic minerals | <10 Ka |
| 54 : Coal and other mine fire minerals (see also #51 and #56) | |
| 55 : Anthropogenic mine minerals |
Type Occurrence of Hexahydrite
General Appearance of Type Material:
Seams (to about 1 cm thick) and scattered patches in altered rock. A somewhat coarse columnar structure or sometimes delictae fibrous. No distinct crystals observed.
Place of Conservation of Type Material:
No designated type specimen.
Other Language Names for Hexahydrite
Relationship of Hexahydrite to other Species
Member of:
Other Members of Hexahydrite Group:
| Bianchite | Zn(SO4) · 6H2O | Mon. 2/m : P2/m |
| Chvaleticeite | Mn(SO4) · 6H2O | Mon. 2/m : B2/b |
| Ferrohexahydrite | FeSO4 · 6H2O | Mon. 2/m : B2/b |
| Moorhouseite | Co(SO4) · 6H2O | Mon. 2/m : B2/b |
| Nickelhexahydrite | Ni(SO4) · 6H2O | Mon. 2/m : B2/b |
Common Associates
Associated Minerals Based on Photo Data:
| 7 photos of Hexahydrite associated with Epsomite | MgSO4 · 7H2O |
| 6 photos of Hexahydrite associated with Betpakdalite-NaCa | [Na2(H2O)17Ca(H2O)6][Mo6+8As5+2Fe3+3O34(OH)3] |
| 6 photos of Hexahydrite associated with Bandylite | Cu[B(OH)4]Cl |
| 6 photos of Hexahydrite associated with Blödite | Na2Mg(SO4)2 · 4H2O |
| 6 photos of Hexahydrite associated with Nitratine | NaNO3 |
| 5 photos of Hexahydrite associated with Pickeringite | MgAl2(SO4)4 · 22H2O |
| 4 photos of Hexahydrite associated with Native Sulphur | S8 |
| 4 photos of Hexahydrite associated with Aubertite | CuAl(SO4)2Cl · 14H2O |
| 3 photos of Hexahydrite associated with Metavoltine | K2Na6Fe2+Fe3+6O2(SO4)12 · 18H2O |
| 2 photos of Hexahydrite associated with Alunogen | Al2(SO4)3 · 17H2O |
Related Minerals - Strunz-mindat Grouping
| 7.CB. | Sarvodaite | Al2(SO4)3 · 5H2O |
| 7.CB.02 | Voudourisite | CdSO4 · H2O |
| 7.CB.05 | Szmikite | MnSO4 · H2O |
| 7.CB.05 | Szomolnokite | FeSO4 · H2O |
| 7.CB.05 | Cobaltkieserite | CoSO4 · H2O |
| 7.CB.05 | Dwornikite | Ni(SO4) · H2O |
| 7.CB.05 | Kieserite | MgSO4 · H2O |
| 7.CB.05 | Poitevinite | (Cu,Fe)SO4 · H2O |
| 7.CB.05 | Gunningite | ZnSO4 · H2O |
| 7.CB.07 | Sanderite | MgSO4 · 2H2O |
| 7.CB.10 | Bonattite | CuSO4 · 3H2O |
| 7.CB.12 | Belogubite | CuZn(SO4)2 · 10H2O |
| 7.CB.15 | Drobecite | CdSO4 · 4H2O |
| 7.CB.15 | Aplowite | (Co,Mn,Ni)SO4 · 4H2O |
| 7.CB.15 | Cranswickite | MgSO4 · 4H2O |
| 7.CB.15 | Rozenite | FeSO4 · 4H2O |
| 7.CB.15 | Starkeyite | MgSO4 · 4H2O |
| 7.CB.15 | Ilesite | Mn2+(SO4) · 4H2O |
| 7.CB.15 | Boyleite | ZnSO4 · 4H2O |
| 7.CB.20 | Siderotil | FeSO4 · 5H2O |
| 7.CB.20 | Jôkokuite | MnSO4 · 5H2O |
| 7.CB.20 | Pentahydrite | MgSO4 · 5H2O |
| 7.CB.20 | Chalcanthite | CuSO4 · 5H2O |
| 7.CB.25 | Chvaleticeite | Mn(SO4) · 6H2O |
| 7.CB.25 | Nickelhexahydrite | Ni(SO4) · 6H2O |
| 7.CB.25 | Bianchite | Zn(SO4) · 6H2O |
| 7.CB.25 | Moorhouseite | Co(SO4) · 6H2O |
| 7.CB.25 | Ferrohexahydrite | FeSO4 · 6H2O |
| 7.CB.30 | Retgersite | NiSO4 · 6H2O |
| 7.CB.35 | Zincmelanterite | (Zn,Cu,Fe)SO4 · 7H2O |
| 7.CB.35 | Melanterite | Fe2+(H2O)6SO4 · H2O |
| 7.CB.35 | Alpersite | (Mg,Cu)(SO4) · 7H2O |
| 7.CB.35 | Bieberite | CoSO4 · 7H2O |
| 7.CB.35 | Boothite | CuSO4 · 7H2O |
| 7.CB.35 | Mallardite | MnSO4 · 7H2O |
| 7.CB.40 | Epsomite | MgSO4 · 7H2O |
| 7.CB.40 | Goslarite | ZnSO4 · 7H2O |
| 7.CB.40 | Morenosite | NiSO4 · 7H2O |
| 7.CB.45 | Meta-alunogen | Al2(SO4)3 · 12H2O |
| 7.CB.45 | Alunogen | Al2(SO4)3 · 17H2O |
| 7.CB.50 | Aluminocoquimbite | Al2Fe2(SO4)6(H2O)12 · 6H2O |
| 7.CB.50 | Lazaridisite | 3CdSO4 · 8H2O |
| 7.CB.52 | Pararaisaite | CuMg[Te6+O4(OH)2] · 6H2O |
| 7.CB.55 | Paracoquimbite | Fe4(SO4)6(H2O)12 · 6H2O |
| 7.CB.55 | Rhomboclase | (H5O2)Fe3+(SO4)2 · 2H2O |
| 7.CB.55 | Raisaite | CuMg[Te6+O4(OH)2] · 6H2O |
| 7.CB.55 | Coquimbite | AlFe3(SO4)6(H2O)12 · 6H2O |
| 7.CB.57 | Caichengyunite | Fe2+3Al2(SO4)6 · 30H2O |
| 7.CB.60 | Kornelite | Fe2(SO4)3 · 7H2O |
| 7.CB.65 | Quenstedtite | Fe2(SO4)3 · 11H2O |
| 7.CB.70 | Lausenite | Fe2(SO4)3 · 5H2O |
| 7.CB.75 | Römerite | Fe2+Fe3+2(SO4)4 · 14H2O |
| 7.CB.75 | Lishizhenite | ZnFe2(SO4)4 · 14H2O |
| 7.CB.80 | Ransomite | CuFe2(SO4)4 · 6H2O |
| 7.CB.85 | Dietrichite | (Zn,Fe2+,Mn2+)Al2(SO4)4 · 22H2O |
| 7.CB.85 | Halotrichite | FeAl2(SO4)4 · 22H2O |
| 7.CB.85 | Apjohnite | Mn2+Al2(SO4)4 · 22H2O |
| 7.CB.85 | Redingtonite | (Fe2+,Mg,Ni)(Cr,Al)2(SO4)4 · 22H2O |
| 7.CB.85 | Pickeringite | MgAl2(SO4)4 · 22H2O |
| 7.CB.85 | Bílinite | Fe2+Fe3+2(SO4)4 · 22H2O |
| 7.CB.85 | Wupatkiite | (Co,Mg,Ni)Al2(SO4)4 · 22H2O |
| 7.CB.90 | Meridianiite | MgSO4 · 11H2O |
Fluorescence of Hexahydrite
Fluoresces and phosphoresces dull cream white (Sterling Mine, Ogdensburg, NJ, USA).
Other Information
Thermal Behaviour:
Heated in a closed tube it yields a large amount of water of neutral pH. Before the blowpipe, on charcoal, it swells and emits bubbles of vapour, but does not melt; and leaves an infusible mass, of neutral pH. When moistened with a solution of cobalt nitrate and reignited the mass becomes pink.
Notes:
Dissolves readily in cold water, giving a clear solution with a bitter salty taste. Adding ammonium chloride, this solution does not give a precipitate, but when a solution of sodium phosphate is added to the ammoniacal solution, a large amount of white precipitate of ammonium-magnesium phosphate is produced. Adding HCl and barium chloride to the starting aqueous solution produces abundant white precipitate of barium sulphate.
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Hexahydrite
mindat.org URL:
https://www.mindat.org/min-1891.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
References for Hexahydrite
Reference List:
Spencer, L. J. (1913) A (sixth) list of new mineral names. Mineralogical Magazine and Journal of the Mineralogical Society, 16 (77) 352-378 doi:10.1180/minmag.1913.016.77.09
Robson, Homer Louis (1927) The system MgSO4·H2O from 68 to 240°. Journal Of The American Chemical Society, 49 (11). 2772-2783 doi:10.1021/ja01410a016
Zalkin, A., Ruben, H., Templeton, D. H. (1964) The crystal structure and hydrogen bonding of magnesium sulfate hexahydrate. Acta Crystallographica, 17 (3) 235-240 doi:10.1107/s0365110x64000603
Nawaz, R. (1973) Nickel-hexahydrite from Tasmania, Australia. Mineralogical Magazine, 39 (302) 246-247 doi:10.1180/minmag.1973.039.302.15
Mitchell, Richard S. (1978) Minerals Which Alternate with the Seasons. Rocks & Minerals, 53 (4) 174-175 doi:10.1080/00357529.1978.11762746
Hon, Ken, Orr, Tim (2011) Hydrothermal hexahydrite spherules erupted during the 2008–2010 summit eruption of Kīlauea Volcano, Hawai`i. Bulletin of Volcanology, 73 (9). 1369-1375 doi:10.1007/s00445-011-0484-x
Lopez-Reyes, G., Sobron, P., Lefebvre, C., Rull, F. (2014) Multivariate analysis of Raman spectra for the identification of sulfates: Implications for ExoMars. American Mineralogist, 99 (8) 1570-1579 doi:10.2138/am.2014.4724
Localities for Hexahydrite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Antarctica | |
| Watanuki/ Torii/ Nishiyama/ Morikawa |
| Barczuk A. & Tatur A. 2003: BIOGENIC ... |
| Barczuk et al. (2003) | |
Argentina | |
| Márquez-Zavalía et al. (1995) +1 other reference |
| Peterson (2011) |
| Bengochea et al. (1998) |
Australia | |
| Ashley et al. (1999) |
| Thienenkamp (2004) |
| Sielecki (1988) +1 other reference |
| Sielecki (1988) | |
| Harris et al. (2003) |
| Rob Fitzpatrick et al. (2010) |
| Raven et al. (2010, August) |
| Mark Raven |
| Wilson et al. (2014) |
| Mills et al. (2010) | |
Austria | |
| Götzinger et al. (2009) |
| J. Mörtl: Carinthia II 192./112.:329-330 (2002) |
| Niedermayr et al. (2007) |
| Strasser (1989) |
| Strasser (1989) | |
| Strasser (1989) |
| Bojar (2007) |
| Postl et al. (2014) |
| Postl et al. (2019) |
| Taucher (1996) |
| NIEDERMAYR et al. (1995) |
| Meixner (1977) +1 other reference |
| Kolitsch et al. (2006) |
| Exel (1993) |
Belgium | |
| Hatert et al. (2002) |
Bulgaria | |
| Младенова et al. (2007) |
Canada | |
| Kohut et al. (1993) | |
| Renaut et al. (1994) |
| Peatfield (n.d.) +1 other reference |
| Peatfield (n.d.) +1 other reference |
| Peatfield (n.d.) +2 other references | |
| |
| Peatfield (n.d.) |
| Geological Association of Canada (2014) |
| Battler et al. (2013) |
| Rocks & Min 81:1 pp 24-32 | |
| Zodrow (1989) |
| Gait et al. (1990) |
| Sabina (1974) |
| Sabina (1983) |
| Sabina (2003) |
| Sabina (1983) |
| Last (1990) |
| Greengrass et al. (1999) | |
| Robinson et al. (1992) |
| 150-152. +2 other references | |
| Sabina (1972) |
Chile | |
| Gerhard Möhn collection |
| Collao et al. (2002) |
| Samples analysed by Tony Kampf of LAC ... +1 other reference |
| Kampf et al. (2013) | |
| Pueyo et al. (2021) +1 other reference |
| Cabestrero et al. (2022) | |
China | |
| Shaoxiu (1991) |
| Shaoxiu (1991) |
| Shaoxiu (1991) |
| Shaoxiu (1991) |
| Yang et al. (2016) |
| Bingxiao (1992) |
| Bingxiao (1992) |
| Kaiyin Bai and Zhaoxin Han (2007) |
Costa Rica | |
| Ulloa et al. (2018) |
Cyprus | |
| Ng +1 other reference |
Czech Republic | |
| Palache et al. (1951) +1 other reference |
| Jirásek (2001) |
| Lapis 2002 (7/8) |
| Sejkora et al. (2019) |
| Matýsek et al. (2023) |
| Matýsek et al. (2014) |
| Matýsek et al. (2014) |
| Jirásek et al. (2011) |
| Matýsek et al. (2022) |
| Matýsek et al. (2022) | |
| Matýsek D. (1999) |
| Matýsek et al. (2024) |
| Hofmanovi P. a R.: Příspěvek k ... |
| Dokoupilova P. |
| Hršelová et al. (2013) | |
| Petr Němeček (2007) |
| Kruťa T. (1944) +1 other reference |
| Petr Pauliš |
Egypt | |
| |
El Salvador | |
| Stoiber et al. (1974) |
France | |
| R. Pierrot |
| R. Pierrot |
| Naze-Nancy Masalehdani et al. (2009) |
| Steven M. Richardson (1978) +1 other reference |
Germany | |
| Gerstenberg (n.d.) |
| |
| Schnorrer (2000) +1 other reference |
| Blaß et al. (1996) +1 other reference |
| Košek (2018) | |
| Blaß et al. (1995) |
| Wittern (2001) |
| Brockt et al. (2001) |
| Wittern (2001) |
| Wittern (2001) |
| Witzke (1992) |
| Witzke et al. (1998) |
| Witzke et al. (1998) |
| Hanneberg et al. (2009) +1 other reference |
| Liebeskind et al. (1992) |
Greece | |
| |
| |
| Rieck (n.d.) | |
| Blaß et al. (1998) |
| Lapis et al. (1999) | |
| Rieck (n.d.) | |
Greenland | |
| Petersen et al. (1993) |
Hungary | |
| Sándor et al. (2005) |
| Szakáll et al. (2008) |
| Koch (1985) |
| www.minerals- of-the- carpathians.eu (Editor:Szakáll) |
| ACTA MIN. PETR. Suppl. Tomus XXXVIII. |
| ACTA MIN. PETR. Suppl. Tomus XXXVIII. | |
| Sánoor Szakáll et al. (1997) |
| Szakáll: Minerals of Rudabánya +1 other reference | |
| Szakáll: Minerals of Rudabánya +1 other reference | |
| Sándor et al. (2005) | |
| ACTA MIN. PETR. Suppl. Tomus XXXVIII. |
| HOM Collection 2005 |
| Sándor et al. (2005) | |
| ACTA MIN. PETR. Suppl. Tomus XXXVIII. +1 other reference |
| ACTA MIN. PETR. Suppl. Tomus XXXVIII. |
| Sándor et al. (2005) |
| Farkas et al. (2009) |
| Geoda - Journal of the Hungarian ... |
| Sándor et al. (2005) |
Iceland | |
| Balić-Žunić et al. (2016) |
| Balić-Žunić et al. (2025) |
India | |
| Equeenuddin (2015) |
Iran | |
| Khorasanipour (2015) |
Italy | |
| D'Angeli et al. (2018) |
| Scacchetti et al. (2023) |
| Galliano et al. (2022) +1 other reference |
| Piccoli et al. (2007) |
| De Michele (1974) |
| D'Angeli et al. (2018) |
| Conticini F. et al. (1980) +1 other reference |
| C.L. Garavelli (1957) |
| Luetcke (n.d.) |
| Sabelli et al. (1984) |
| D'Angeli et al. (2018) |
Japan | |
| Seki et al. (1987) |
| Nambu et al (1980) |
| SHIMOBAYASHI et al. (2011) |
Kenya | |
| Bowell et al. (1996) |
Mexico | |
| Gazquez et al. (1) |
| |
| Panczner (1987) | |
| Yta et al. (2005) |
Morocco | |
| S. Weiß: Lapis 31 (7/8) |
Namibia | |
| Dill et al. (2002) |
Nicaragua | |
| Hynek et al. (2013) |
North Macedonia | |
| Alois Lechner collection (analysed by Günter Blass) |
| Kolitsch et al. (2018) |
Norway | |
| Neumann (1985) |
| Neumann (1985) |
| Garmo (1974) +1 other reference |
| Raade (1989) |
| Raade (1973) +2 other references |
| Neumann (1985) |
| Raade et al. (1990) |
| Witsø (1994) +1 other reference |
| Jørgensen et al. (2001) |
| Jørgensen et al. (2001) |
| Raade (1989) |
| Ellingsen (1989) |
Peru | |
| Diaby et al. (2006) |
Poland | |
| powder rtg. 2007 |
| Cabała et al. (2008) |
| Ciesielczukk |
| Stepisiewicz (1983) |
| Lukasz Kruszewski 2005: Minerals arising in cause of underground fires of "Skalny" coal mine dump in Laziska (unpublished) +1 other reference |
| Kruszewski (2013) |
| Kruszewski (2013) |
| Bril et al. (2008) |
| Kruszewski (2013) | |
| Ł. Kruszewski PXRD data (to be published soon) |
| Kruszewski et al. (2018) |
Portugal | |
| Joana Ribeiro et al. (2013) |
| Álvarez-Valero et al. (2008) |
| Oliveira et al. (2024) |
Romania | |
| Apopei et al. (2014) |
| Januszewska et al. (2023) |
| A. Januszewska PXRD data / Januszewska et al. (in preparation) | |
Russia | |
| Cesnokov et al. (1998) |
| [Der Aufschluss 1995:4 p163-180] +1 other reference |
| [Der Aufschluss 1995:4 p163-180] | |
| |
| Indolev L N et al. (1971) |
| Antonov A.A. [Антонов А.А.] (2003) |
| Kasatkin et al. (2014) |
Senegal | |
| Montoroi (1995) |
Slovakia | |
| Koděra (1986) |
| Ďuďa (1993) |
| Koděra (1986) |
| Števko M. et al. (2017) |
| Myšľan P. et al. (Slovenská republika) |
South Africa | |
| Cairncross et al. (1995) |
South Korea | |
| Pavel M. Kartashov's analytical data |
Spain | |
| Rewitzer et al. (2020) |
| Schnorrer (2000) |
| Valente et al. (2013) |
| Valente et al. (2013) | |
| Valente et al. (2013) | |
| MinMag 67 (2) |
| Romero et al. (2006) +1 other reference |
| Vizcayno et al. (1995) |
| Fernando Gascón collection +1 other reference |
| Fernando Gascón collection | |
| Calvo (2008) |
| Campeny et al. (2023) |
| Buey et al. (2025) |
| Buey et al. (2025) | |
| Sanz-Montero et al. (2019) +1 other reference | |
| Garcia-Guinea et al. (2018) |
| Joan Abella i Creus (Joanabellacreus@gmail.com) |
| Crespo López et al. (2023) |
| Benavente +2 other references |
| Benavente et al. (2018, September) |
Switzerland | |
| Romani E. (2000) |
| Romani (2000) +1 other reference |
| Philipp R. (1983) |
| Stalder et al. (1998) |
| Cannon (2005) +1 other reference |
| Perroud et al. (1987) +2 other references |
| Stalder et al. (1998) +1 other reference |
| Stalder et al. (1998) |
| Stalder et al. (1998) |
Tunisia | |
| Smykatz-Kloss et al. (2010) |
Turkey | |
| Helvaci (1998) |
| Çelik Karakaya M & Karakaya N (2011) | |
| van Doesburg et al. (1982) |
Turkmenistan | |
| |
UK | |
| Smith et al. (2014) |
| Kemp et al. (2016) | |
| Livingstone et al. (1983) |
Ukraine | |
| Popov S.P. Mineralogy of the Crimea (1938) |
| Deyak M.A. Modern water-chemogenic ... |
| Palache et al. (1951) |
| A. Tischenko. Minerals of the Crimea - ... |
| Dvoichenko P.A. The minerals of Crimea (1914) |
| Möckel et al. (1992) |
| Dobrovolskaya T.I. (2004) |
USA | |
| Anthony et al. (1995) |
| Graeme (1981) +1 other reference | |
| Wenrich (1988) +1 other reference | |
| Gornitz (2004) |
| Anthony et al. (1976) +1 other reference |
| Howard (2007) |
| Committee et al. (1989) |
| Committee et al. (1989) | |
| Adams et al. (2014) |
| Adams et al. (2014) |
| a spectroscopic exploration. Economic ... +1 other reference |
| Schlocker (1974) +2 other references |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Zielinski et al. (2001) |
| Januzzi (1976) |
| Hon et al. (2011) |
| |
| Cerato |
| Heinrich et al. (2004) |
| Buck et al. (2006) |
| USGS Prof Paper 860 +1 other reference |
| Castor et al. (2004) |
| Castor et al. (2004) |
| Castor et al. (2004) | |
| Castor et al. (2004) |
| Dunn (1995) |
| Caldwell (2018) |
| 2008 New Mexico Mineral Symposium ... |
| Patrick Haynes collection |
| Januzzi +1 other reference |
| Carlson (2015) |
| Carlson (2015) | |
| Carlson (2015) |
| King (n.d.) |
| Ernest H. Carlson. Jerret Whitford. ... | |
| Carlson (2015) |
| Carlson (2015) |
| gsa.confex.com (2002) |
| Carlson (2015) |
| Carlson (2015) |
| Carlson (2015) |
| Carlson (2015) |
| Carlson (2015) |
| Carlson (2015) | |
| Gold (2006, October) |
| Barnes (1973) +2 other references |
| www.jonesgeo.com (2011) |
| Coskren et al. (2000) |
| Journal of Geosciences (2015) +2 other references |
| USGS TEI #514 |
| Dietrich (1990) |
| Dietrich (1990) |
| Dietrich (1990) | |
| Dietrich (1990) |
| Dietrich (1990) |
| Dietrich (1990) | |
| Dietrich (1990) |
| Dietrich (1985) |
| Dietrich (1990) | |
| Dietrich (1990) |
| Kilburn et al. (1996) |
| Cannon (1975) |
| Cannon (1975) |
| Luetcke (n.d.) |
Uzbekistan | |
| Uklonskiy et al. (1964) +1 other reference |
| Slyusareva (1964) |
Venezuela | |
| Franco Urbani (2009) |
Quick NavTopAbout HexahydriteUnique IdentifiersIMA Classification Classification Mineral SymbolsPronunciation Physical Properties Optical Data Chemistry Crystallography Crystal StructureX-Ray Powder DiffractionGeological EnvironmentType Occurrence Other LanguagesRelationshipsCommon AssociatesStrunz-MindatFluorescence Other InformationInternet Links References Localities Locality List




 symbol to view information about a locality.
The
symbol to view information about a locality.
The
Nabo Ravine, Teruel, Teruel, Aragon, Spain