SUMMARY
Honaucins A–C were isolated from the cyanobacterium Leptolyngbya crossbyana which was found overgrowing corals on the Hawaiian coast. Honaucin A consists of (S)-3-hydroxy-γ-butyrolactone and 4-chlorocrotonic acid which are connected via an ester linkage. Honaucin A and its two natural analogs exhibit potent inhibition of bioluminescence, a quorum sensing-dependent phenotype, in Vibrio harveyi BB120 as well as of lipopolysaccharide-stimulated nitric oxide production in the murine macrophage cell line RAW264.7. The decrease in nitric oxide production was accompanied by a decrease in the transcripts of several pro-inflammatory cytokines, most dramatically interleukin-1β. Synthesis of honaucin A as well as a number of analogs and subsequent evaluation in anti-inflammation and quorum sensing inhibition bioassays revealed the essential structural features for activity in this chemical class, and provided analogs with greater potency in both assays.
INTRODUCTION
Marine cyanobacteria have emerged as one of the richest sources of biologically active and structurally unique natural products (Tidgewell et al., 2010). To date, there has been a strong focus on the anticancer properties of cyanobacterial natural products, perhaps due in part from funding priorities in biomedical research and in part from a competitive advantage that these cytotoxic metabolites may confer on their producers by deterring grazing (Nagle and Paul, 1999). Regardless, it is likely that continued interrogation of this natural product rich group will result in the discovery of compounds with applications in other therapeutic areas such as management of inflammation, infection, and neurological diseases.
Inflammation, in particular, is an attractive therapeutic target due to its pervasive impacts on human health. In addition to well-known chronic inflammatory disorders such as rheumatoid arthritis and asthma, it is now recognized that many diseases not previously thought to have an autoimmune basis do involve inflammation, including cancer, heart diseases, skin diseases, and disorders of the bowel (Grivennikov et al., 2010; Tousoulis et al., 2011; Cheung et al., 2011). The ability to effectively treat chronic inflammation is thus of great importance both from the perspective of disease prevention and management as well as reduction of health care costs. However, the most commonly prescribed anti-inflammatory drug classes, corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs), both have undesirable side effects including hypertension and osteoporosis in the case of the former and gastrointestinal irritation and renal damage in the latter (Moghamdam-Kia and Werth, 2010; Conaghan, 2011). Thus, an unmet medical demand exists for novel anti-inflammatory agents that exert their effects through different modes of action; the natural products of marine cyanobacteria may offer one possible source of such compounds. Indeed, diverse marine organisms including marine cyanobacteria have already been found to be sources of anti-inflammatory metabolites which operate by novel mechanisms (Terracciano et al., 2006; Gautam and Jachak, 2009; Villa and Gerwick, 2010; Flachsmann et al, 2010).
The ability of small molecules to regulate quorum sensing (QS) among pathogenic microorganisms represents a second relatively unexplored area of drug discovery from marine cyanobacteria. QS is a population density-based signaling process through which prokaryotes coordinate diverse cellular responses including initiation of sporulation, swarming, horizontal gene transfer, production or repression of virulence factors and other secondary metabolites (to initiate pathogenic or encourage mutualistic interactions), bioluminescence, and biofilm formation (Pappas and Winans, 2003; Zhang et al., 2002; Ni et al., 2009a). QS is regulated by the production and chemoreception of signaling molecules known as ‘autoinducers’. When the density of an organism is sufficient for the concentration of the autoinducer to surpass a threshold, gene expression related to the above physiological responses is triggered (Teng et al., 2011). Because QS can be a factor in the pathogenicity of infectious microorganisms, inhibitors of this process have garnered interest for their potential therapeutic applications. QS inhibition of pathogenic microbes is especially appealing because it has the potential to impair the ability of the pathogen to cause disease yet is not overtly lethal and thus is unlikely to lead to the development of resistant phenotypes (Galloway et al, 2011).
Several marine natural products, possessing anti-inflammatory properties, have been reported from marine invertebrates such as corals and sponges as well as from marine microorganisms (Terracciano et al., 2006; Gautam and Jachak, 2009; Villa and Gerwick, 2010; Flachsmann et al, 2010). With respect to marine microorganisms, which in many cases are likely the actual producers of the anti-inflammatory compounds isolated from invertebrates, it is interesting to speculate that they may have acquired the ability to produce anti-inflammatory compounds in response to evolutionary pressures to overcome the innate immune response mounted by their hosts (Villa and Gerwick, 2010; Ogier et al., 2010; McFail-Ngai et al, 2010).
Recent studies of bacterial communication and host-bacteria interaction reveal that bacterial QS modulators have diverse and sometimes contradictory effects on host cell physiology. Under different circumstances, these can include either inhibition or stimulation of the immune response, suggesting that microbial QS signaling molecules are key modulators of intra- and inter-kingdom interactions (Rumbaugh and Kaufmann, 2011). However the exact relationship between bacterial QS and host immunity is still unclear. Therefore, identification of natural products with the capacity to both modulate bacterial QS and host immune responses will provide useful chemical tools for study of the relationships between these two ecological phenomena as well as provide new lead molecules for drug discovery.
In these regards, we have evaluated the extracts and metabolites of marine cyanobacteria as potential inhibitors of both the mammalian innate immune system and bacterial QS signaling. As a result, we describe here a group of newly discovered natural products with both of these biological properties, honaucins A–C (1–3). These were isolated from the Hawaiian cyanobacterium Leptolyngbya crossbyana which was found overgrowing coral reefs in the Hōnaunau Bay of Kona (Smith et al., 2008). The structures of honaucins A–C were elucidated by standard spectroscopic techniques, and confirmed by total organic synthesis. Each of these new metabolites inhibited nitric oxide (NO) production as well as the expression of selected pro-inflammatory cytokines in RAW264.7 murine macrophage cells stimulated with lipopolysaccharide (LPS). In addition, these new natural products were found to inhibit QS signaling-dependent phenotypes in Vibrio harveyi BB120 and Escherichia coli JB525. Synthesis of a mini-panel of honaucin analogs was followed by evaluation in the appropriate bioassays, and revealed several critical structural features for biological activity in this structure class. Moreover, this effort resulted in the discovery of honaucin derivatives with improved anti-inflammatory and QS inhibitory properties.
RESULTS
Isolation and Structure Elucidation of Honaucins A–C
The colonial cyanobacterium Leptolyngbya crossbyana (HI09-1) was collected in January 2009 by SCUBA from Hōnaunau reef off the island of Hawaii and extracted repetitively with 2:1 CH2Cl2-CH3OH to yield 1.7 g of crude extract. A portion of the organic extract (1.4 g) was subjected to silica gel vacuum-liquid chromatography (VLC) using a stepwise gradient of hexanes/EtOAc/MeOH to produce nine fractions (A–I). Fraction E, eluting with 40% hexanes in EtOAc, was found to potently inhibit production of NO in LPS-stimulated macrophages. This fraction was subjected to bioactivity-guided fractionation using silica Sep-Pak chromatography and RP HPLC to afford three pure anti-inflammatory compounds, honaucins A (1, 5.4 mg, 0.39%), B (2, 1.4 mg, 0.10%), and C (3, 1.1 mg, 0.08%).
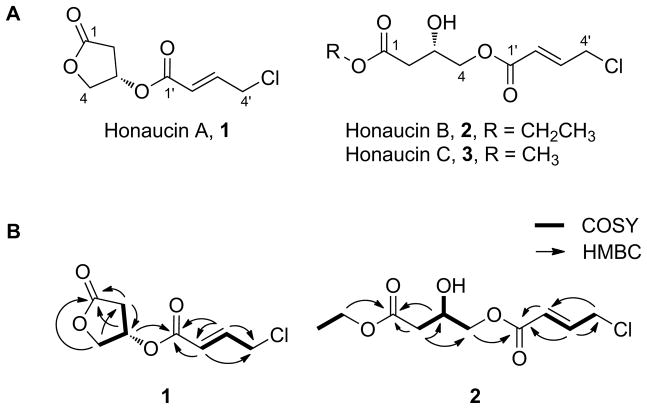
Honaucin A (1) was obtained as a colorless oil and its HR ESIMS showed a molecular ion cluster at m/z 205.0262/207.0240 in a ratio of 3:1, indicating the presence of one chlorine atom (Figure S1). The molecular formula of 1 was determined as C8H9ClO4 by HR ESITOFMS ([M+H]+ m/z 205.0262), and the IR spectrum displayed absorption bands at 1784 and 1724 cm−1 for the presence of γ-lactone and α,β-unsaturated ester functionalities. The 1H and 13C NMR spectra of 1 displayed two downfield quaternary carbons (δC 174.5 and 165.0), two olefinic protons (δH 7.06 and 6.13), an O-substituted methine (δH 5.53), and an O-substituted methylene (δH 4.55 and 4.42) (Table 1; Figure S1). NMR data from gCOSY, gHSQC and gHMBC experiments allowed construction of β-O-substituted γ-butyrolactone and 4-chlorocrotonic acid moieties. Finally, a gHMBC correlation from H-3 to C-1′ revealed a connection between these partial structures, thus providing the planar structure of honaucin A (1) (Figure 1).
Table 1.
NMR Spectroscopic Data for Honaucin A–C (1–3) in CDCl3a
| 1 | 2 | 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Position | δC, mult. | δH, multi (J in Hz) | COSY | HMBCb | δC, multi | δH, multi (J in Hz) | COSY | HMBCb | δC, multi | δH, multi (J in Hz) |
| 1 | 174.5, qC | 172.2, qC | 172.2, qC | |||||||
| 2a | 34.8, CH2 | 2.91, dd (18.5, 6.7) | 2b, 3 | 1, 4 | 38.0, CH2 | 2.57, s | 3 | 1, 3 | 38.0, CH2 | 2.59, s |
| 2b | 2.67, d (18.5) | 2a | 1, 3, 4 | 2.56, d (3.46) | 3 | 2.57, d (2.82) | ||||
| 3 | 70.3, CH | 5.53, t (5.7) | 2a, 4a | 1, 4, 1′ | 66.5, CH | 4.32, m | 2, 4 | 2, 4 | 66.5, CH | 4.31, m |
| 4a | 73.1, CH2 | 4.55, dd (11.1, 4.7) | 3, 4b | 67.4, CH2 | 4.22, m | 3 | 2, 3 | 67.4, CH2 | 4.21, m | |
| 4b | 4.42, d (11.1) | 4a | 1, 2, 3 | |||||||
| 4-OH | 3.14, brs | 4 | 3.08, brs | |||||||
| 5 | 61.3, CH2 | 4.18, m | 6 | 1 | 61.3, CH3 | 3.74, s | ||||
| 6 | 14.4, CH3 | 1.28, t (6.70) | 5 | 5 | ||||||
| 1′ | 165.0, qC | 165.7, qC | 165.7qC | |||||||
| 2′ | 122.9, CH | 6.13, d (15.4) | 3′, 4′ | 1′, 3′, 4′ | 123.6, CH | 6.16, d (15.4) | 3′, 4′ | 1′, 3′, 4′ | 123.6, CH | 6.16, d (15.2) |
| 3′ | 144.1, CH | 7.06, dt (15.4, 5.9) | 2′, 4′ | 1′, 2′, 4′ | 142.9, CH | 7.04, dt (15.3, 5.9) | 2′, 4′ | 1′, 2′, 4′ | 142.9, CH | 7.03, dt (15.3, 5.8) |
| 4′ | 42.5, CH2 | 4.20, d (5.9) | 3′ | 1′, 2′, 3′ | 42.6, CH2 | 4.19, m | 3′ | 2′, 3′ | 42.6, CH2 | 4.16, m |
Measured at 600 MHz for 1H NMR and 150 MHz for 13C NMR.
From 1H to the indicated 13C.
Figure 1. Structures of Honaucins A–C (1–3).
(A) Structures of honaucins A–C (1–3); (B) Structure elucidation of 1 and 2 based on NMR data; see also Figures S1–S3.
Honaucin B (2) showed a similar isotope pattern to 1 by positive ion HR ESITOFMS ([M+Na]+ m/z 273.0499) indicating a molecular formula of C10H15ClO5 (Figure S2). The IR spectrum of 2 displayed absorption bands at 3454, 1724 and 1659 cm−1, indicating the presence of alcohol, α,β-unsaturated ester and isolated ester functionalities, respectively. The 1H and 13C NMR spectrum of 2 showed several resonances similar to those in 1 (Table 1; Figure S2), thus confirming the presence of 4-chlorocrotonic acid and a 3,4-di-O-substituted butanoic acid moiety (Figure 1). A gHMBC correlation from H-4 to C-1′ indicated an ester linkage between these positions whereas a correlation from H-5 to C-1 indicated that an ethyl group was linked to C-1 via an ester bond.
Honaucin C (3, m/z 259.0346 for C9H13ClO5) also displayed an isotope pattern consistent with one chlorine atom by positive ion HR ESITOFMS (Figure S3). The 1D and 2D NMR spectra of 3 were almost identical to those of 2, although the methyl triplet (H3-6, δH 1.28) and the methylene (H2-5, δH 4.18) in the ethyl group in 2 were replaced by a methyl singlet (H3-5, δH 3.74) in 3 (Table 1; Figure S3). Finally, gHMBC correlations from H-5 to C-1 and from H-4 to C-1′ led to the planar structure of honaucin C (3).
The absolute configuration at C-3 in 1 was determined as S by comparing the optical rotation of 1 ([α]D22 −80.5) with literature values of (R/S)-3-hydroxy-γ-butyrolactone (Uchikawa et al., 1988). This assignment was confirmed by total synthesis of honaucin A as well as its C-3 epimer (17). Synthetic (−)-honaucin A was prepared by Steglich esterification of 4-chlorocrotonic acid (7) and (S)-3-hydroxy-γ-butyrolactone (4) (see Supplemental Experimental Procedures). Similarly, epi-honaucin A (17) was synthesized from 4-chlorocrotonic acid (7) and (R)-3-hydroxy-γ-butyrolactone (5). Synthetic honaucin A was found to have a specific rotation of −77.5, similar to that of the natural compound, whereas epi-honaucin A (17) showed a specific rotation of +66.0. The absolute configuration at C-3 in metabolites 2 and 3 was also determined as S by comparing the specific rotation of 2 and 3 with that of the literature value for the 4-O-tritylated derivative of ethyl (S)-3,4-dihydroxybutanoate (Prasad et al., 1990).
Anti-inflammatory Activity of Honaucins A–C
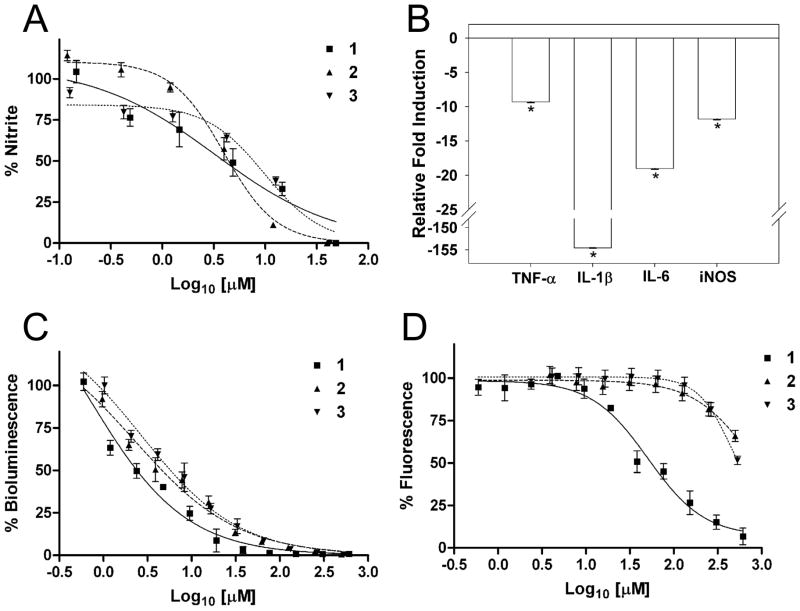
The degree of NO production in LPS-stimulated macrophages provides a measurement of induced inflammation via the activation of TLR4, followed by intracellular signaling via the MyD88 dependent and the MyD88 independent pathways and the subsequent activation of the inducible nitric oxide synthase gene (iNOS). We exploited these pathways in an initial phenotypic screen to assess the anti-inflammatory potential of honaucins A–C (1–3). NO production by LPS-stimulated RAW264.7 murine macrophages was inhibited by compounds 1–3 with IC50 values of 4.0, 4.5 and 7.8 μM, respectively. By contrast, the IC50 value for the cytotoxicity of honaucin A in the cells was greater than 49 μM, giving a greater than ten-fold therapeutic window between its NO inhibitory effect and cytotoxicity (Figure 2A and Table 2).
Figure 2. Biological Properties of Honaucins A–C (1–3) (mean +/− SD, n=3).
(A) Inhibition of LPS-stimulated NO production on murine macrophages; (B) Downregulation of proinflammatory cytokines in LPS-stimulated murine macrophages by honaucin A, (* p < 0.002 compared to LPS treatment alone); (C) Inhibition of QS signaling in V. harveyi BB120; (D) Inhibition of QS signaling in E. coli JB525; see also Figure S4.
Table 2.
Biological Activities of Natural and Synthetic Honaucin A Derivatives
| Compounds | NO inhibition (IC50 ± SDa, μM) | p values for IC50 values from NO inhibition compared to compound 1 | Cell survival at IC50 of NO inhibition (%) | Cytotoxicity in Raw264.7 cells (IC50, μM) | QS inhibition (IC50 μM) | |
|---|---|---|---|---|---|---|
| V. harveyi | E. coli | |||||
| 1 | 4.0 ± 2.3 | 100 | > 49 | 5.6 | 38.5 | |
| 2 | 4.5 ± 1.0 | 0.7473 | 96.8 | 16 | 17.6 | 908 |
| 3 | 7.8 ± 1.4 | 0.0709 | 85.2 | 30 | 14.6 | 576 |
| 4 | 21.0 ± 10.8 | 0.0560 | 100 | > 98 | -d | -d |
| 5 | 70.7 ±15.7 | 0.0019 | 100 | > 98 | -d | -d |
| 6 | -b | -c | -b | > 98 | -d | -d |
| 7 | 28.0 ± 4.3 | 0.0010 | 95.5 | 80 | 72.5 | 152 |
| 8 | -b | -c | -b | > 81 | -d | -d |
| 9 | -b | -c | -b | > 116 | -d | -d |
| 10 | -b | -c | -b | > 116 | -d | -d |
| 11 | -b | -c | -b | > 100 | -d | -d |
| 12 | 55.8 ± 5.9 | 0.0001 | 95.0 | > 67 | -d | -d |
| 13 | 11.6 ± 4.3 | 0.0540 | 100 | > 288 | 167 | > 304 |
| 14 | 3.5 ± 0.5 | 0.7316 | 94.7 | 35 | 10.5 | -e |
| 15 | 1.2 ± 0.2 | 0.1036 | 96.4 | 10 | 1.2 | -e |
| 16 | 9.7 ± 3.6 | 0.0819 | 80.0 | 56.5 | -d | -d |
| 17 | 39.3 ± 3.7 | 0.0001 | 94.0 | > 49 | 22.1 | 260 |
| 18 | 19.6 ± 5.3 | 0.0015 | 100 | > 49 | 15.9 | 221 |
| 19 | -b | -c | -b | > 49 | -d | -d |
| 20 | 20.8 ± 3.3 | 0.0019 | 100 | > 54 | -d | -d |
| 21 | -b | -c | -b | > 59 | -d | -d |
| 22 | -b | -c | -b | > 59 | -d | -d |
| 23 | 6.2 ± 1.5 | 0.2375 | 100 | > 43 | -d | -d |
| 24 | 4.3 ± 0.6 | 0.8377 | 85.0 | 128 | 168 | 342 |
| 25 | 1.5 ± 0.0 | 0.0001 | 91.7 | 22 | 0.24 | 0.51 |
| 26 | 0.9 ± 0.1 | 0.0801 | 100 | 23 | 0.13 | 0.70 |
Standard deviation was calculated from three technical replicates.
No activity was observed with the compound up to the highest concentration tested (30 μg/mL).
Inhibition of NO production in LPS-stimulated Raw 264.7 cells did not reach to 50% at the highest test concentration (30 μg/mL) and the p value was not able to be calculated.
No activity was observed with the compound up to the concentration of 125 μg/mL.
Due to the antibiotic effects of these compounds, their IC50 values could not be reliably measured.
To determine whether decreased NO production in the presence of honaucin A (1) was due to changes at the mRNA level or through radical oxygen scavenging, the antioxidant activity of honaucin A was evaluated. However, no antioxidant activity was observed up to 146 μM (Figure S4). Quantitation of the transcript levels of key pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and iNOS in honaucin A-treated versus untreated LPS-stimulated RAW 264.7 cells was accomplished by quantitative real-time polymerase chain reaction (qRT-PCR). Cells treated with compound 1 had reduced transcript levels of all four cytokines after 6 h of treatment (reduction in transcripts of TNF-α, IL-1β, IL-6 and iNOS were −9.3, −154.6, −19.0 and −11.8 fold, respectively; Figure 2B).
Inhibition of QS Phenotypes by Honaucins A–C
Honaucins A–C (1–3) were also screened for inhibition of QS-regulated phenotypes in two different bacterial species, in part due to the structural resemblance of honaucins A–C to other small lactone microbial signaling molecules. The wild-type strain V. harveyi BB120 uses QS to regulate various phenotypes, including bioluminescence (Henke and Bassler, 2004). Chemical communication by V. harveyi is accomplished via three parallel signaling pathways that utilize the autoinducers HA1-1, CAI-1, and AI-2, respectively (Henke and Bassler, 2004). In the HAI-1 system, membrane-bound histidine kinase (LuxN-type) proteins detect the native autoinducer 3-hydroxybutanoyl-L-homoserine lactone. These are detected on the cell surface and information is relayed into the cell via an intracellular signaling cascade (Freeman et al., 2000; Swem et al., 2008; Swem et al., 2009). Genetic knockouts of the HAI-1 system display a 99.9% reduction in bioluminescence (Henke and Bassler, 2004). All three natural honaucins (1–3) demonstrated a dose-dependent inhibition of bioluminescence with IC50 values of 5.6, 17.6 and 14.6 μM, respectively (Figure 2C), thus indicating the capacity of the honaucins to antagonize this QS system. The γ-butyrolactone functionality is not an essential feature for inhibition of QS-mediated bioluminescence in V. harveyi BB120, although it does have impacts on potency.
A second reporter strain, E. coli strain JB525, was used to further test for anti-QS activity of the honaucins (Andersen et al, 2001). This strain has been engineered to produce the V. fischeri LuxR receptor for acyl homoserine lactones (AHLs). In contrast to the HAI-1 system of V. harveyi where the AHL binds to a membrane bound receptor, the AHL in V. fisheri diffuses into the cell to bind its cytoplasmic receptor for QS initiation (Andersen et al., 2001; Swem et al, 2009). Once activated by exogenously supplied autoinducer (3-oxo-hexanoyl homoserine lactone, OHHL), LuxR positively affects expression of a luxI promoter that initiates production of an unstable green fluorescent protein (GFP). Thus, measurement of GFP fluorescence reflects the quantity of LuxR with bound AHL (Andersen et al., 2001). Honaucin A (1) dose-dependently inhibited production of GFP in E. coli JB525 with an IC50 value of 38.5 μM (Figure 2D). Honaucins B (2) and C (3) were essentially inactive in this assay, (IC50 values of 908 and 576 μM, respectively indicating the importance of the γ-butyrolactone pharmacophore in antagonism of QS-regulated GFP production in E. coli JB525. No growth inhibitory effects were observed for metabolites 1–3 in either assay at the highest concentrations tested (610, 458, and 483 μM, respectively).
Structurally-Modified Honaucin A Derivatives
Within its relatively small structure, honaucin A (1) possesses three distinct functional groups; an (S)-3-hydroxy-γ-butyrolactone, an esterified α,β-unsaturated carboxylate, and a primary halogen atom (Figure 3A). The γ-butyrolactone is a conserved functional group in AHLs, the primary class of QS molecule in Gram negative bacterial signaling. Previous efforts to develop synthetic inhibitors of AHL-mediated QS signaling have mostly retained the homoserine lactone feature (Lowery et al., 2010). However, to our knowledge β-hydroxy-γ-butyrolactones have not previously been evaluated for QS inhibition or anti-inflammatory activity. Similarly, while a series of 4-substituted crotonic acid esters were previously assayed for antifungal activity (Gershon et al., 1976), this structural moiety and related derivatives have not been examined for QS inhibitory or anti-inflammatory activities.
Figure 3. Synthesis of Honaucin A and its Synthetic Analogs.
(A) The two building units of honaucin A (1); (B) Synthetic scheme of honaucin A and its analogs; (C) Synthetic honaucin A analogs and their building units.
Thus, with the goal of identifying structural features responsible for the anti-inflammatory and QS inhibitory activities of the honaucins, we prepared analogs of the subunits forming the honaucins and then combined these in a systematic fashion. Modifications included alterations to the configuration and position of the hydroxy substituent on the γ-butyrolactone ring (4–6, 16–17), presence and position of the double bond, carbon chain length and nature of the halogen atom in the crotonic acid portion (7–15, 18–26) (Figure 3C). The resulting analogs were then assayed in the QS inhibitory and anti-inflammatory assays (Table 2).
Anti-inflammatory Activities of Synthetic Honaucin A Derivatives
Of the three structural subunits representing the lactone portion, (S)-3-hydroxy-γ-butyrolactone (4), (R)-3-hydroxy-γ-butyrolactone (5) and (R/S)-2-hydroxy-γ-butyrolactone (6), the fragment representing natural honaucin A (1) was the most potent (IC50 of 4 = 20.9 μM, Table 2; Figure 4A). Both the configuration and position of the hydroxy group in the lactone moiety is important to the anti-inflammatory potency of these γ-butyrolactones. The second subunit, 4-chlorocrotonic acid (7), showed modest anti-inflammatory activity by itself (IC50= 28.0 μM, Table 2; Figure 4B). The chain extended derivative (12) showed similar activity to 7 whereas modification of the conjugated double bond of 4-chlorocrotonic acid (8, 10, 11) significantly decreased anti-inflammatory activity. The inhibition of NO production by compounds 8, 10 and 11 in LPS-stimulated Raw 264.7 cells did not reach to 50% at the highest test concentration (30 μg/mL), so their IC50 values could not be determined. The presence of a halogen atom at C-4 of crotonic acid (9, 13–15) also led to a dramatic change in anti-inflammatory activity. While crotonic acid (9) displayed no activity up to 116 μM, 4-fluorocrotonic acid (13), 4-bromocrotonic acid (14) and 4-iodocrotonic acid (15) were potent NO inhibitors with IC50 values of 11.6 μM, 3.5 μM and 1.2 μM, respectively (Table 2; Figure 4C). Therefore, the conjugated double bond and halogen atom present in the carboxylic acid moiety appear to be quite important to the anti-inflammatory properties of the honaucins.
Figure 4. Inhibition of LPS-stimulated Production of NO in Murine Macrophages by Honaucin A Analogs and their Structural Units (mean +/− SD, n=3).
(A) Inhibition of LPS-stimulated NO production by 1 compared to hydroxy-γ-butyrolactones 4–6; (B) Inhibition of LPS-stimulated NO production by 1 compared to carboxylic acids 7–12; (C) Inhibition of LPS-stimulated NO production by 1 compared to 4-halogenated crotonic acids 7 and 13–15; (D) Inhibition of LPS-stimulated NO production by 1 compared to selected honaucin A derivatives 24–26; see also Figure S5.
In synthetic analogs of honaucin A containing both the γ-butyrolactone and crotonic acid portions, modifications to the point of attachment between the two subunits [epi-honaucin A (17) and 2-hydroxy honaucin A (18)] generally led to less potent molecules. As shown for compounds 8, 10, and 11, most modifications (19, 21–22) of the double bond portion of the 4-chlorocrotonic acid moiety of honaucin A resulted in decreased anti-inflammatory activity and they did not inhibit 50% NO production in LPS-stimulated murine macrophage at the highest concentration tested (30 μg/mL). The exception to this trend was substitution of the halogen atom; 4′-bromohonaucin A (25) and 4′-iodohonaucin A (26) exhibited improved anti-inflammatory activities with IC50 values of 1.5 μM and 0.8 μM, respectively (Table 2; Figure 4D).
Inhibition of QS Phenotypes by Synthetic Analogs of Honaucin A
The QS-mediated antagonism of V. harveyi bioluminescence by the naturally-occurring analogs honaucins B (2) and C (3) indicated that the butyrolactone functional group was not necessary for activity. The various γ-butyrolactones (4–6) by themselves showed no inhibitory activity in either the V. harveyi BB120 or E. coli JB525 assays, indicating that this moiety by itself was also not sufficient for QS antagonism (Table 2). By contrast, the 4-chlorocrotonic acid (7) subunit retained partial inhibitory activity of V. harveyi bioluminescence with an IC50 of 72.5 μM.
However, modification of the halogen atom at the 4-position of the crotonic acid fragment greatly modulated QS-inhibitory activity. While 4-fluorocrotonic acid (13) had decreased inhibitory activity against V. harveyi BB120 (IC50 = 167 μM), the 4-bromocrotonic acid fragment (14) showed a marked increase in activity (IC50 = 10.5 μM) and 4-iodocrotonic acid (15) exhibited a 60-fold increase in activity (IC50 = 1.2 μM) in comparison with 4-chlorocrotonic acid (7). However, it is noteworthy that both 7 and 14 were mildly antibiotic at high concentrations (antibiotic effects were observed at 14- and 72-fold greater concentrations, when comparing MIC of growth inhibitory effects to QS inhibitory IC50 values, respectively). The most potent crotonic acid fragment, 15, showed no growth inhibitory effects up to 32 μM.
This same trend was observed in the inhibition of GFP production by E. coli JB525. 4-Chlorocrotonic acid (7) inhibited GFP production with an IC50 of 152 μM while 4-fluorocrotonic acid (13) was more than two-fold less active. The brominated and iodinated analogs (14, 15) were the most potent, but antibiotic effects (MIC = 188 and 18 μM, respectively) precluded confident measurements of their IC50 concentrations.
Of derivatives containing both core moieties of the honaucins, 3-epi-honaucin A (17) and 2-hydroxy honaucin A (18) were 3- to 4-fold less active than honaucin A against both sensor strains, suggesting that the 3S attachment represents the optimal position and configuration. Halogen substitution had a dramatic effect on the QS inhibitory activity of the intact honaucin A analogs (Figure 5). Against V. harveyi, the fluorinated derivative (24) was 30-fold less potent whereas the brominated and iodinated analogs (25, 26) were an impressive 23- and 43-fold more active (IC50 = 0.24 μM and 0.13 μM), respectively. A similar trend was observed against the E. coli JB525 sensor strain; 24 was 9-fold less potent than 1 while 25 and 26 possessed IC50 concentrations of 0.51 μM and 0.70 μM (76- and 55-fold more active), respectively. Of derivatives containing both core moieties, antibacterial effects were only observed for compounds 25 and 26, which only modestly affected bacterial growth in both assays (MIC = 251 μM and 422 μM, respectively, against E. coli JB525; MIC = 125 μM and 105 μM, respectively, against V. harveyi BB120).
Figure 5. Inhibition of QS-Signaling by Honaucin A (1), 4′-Fluorohonaucin A (24), 4′-Bromohonaucin A (25) and 4′-Iodohonaucin A (26) (mean +/− SD, n=3).
(A) Inhibition of QS-signaling phenotype in Vibrio harveyi BB120; (B) Inhibition of QS-signaling phenotype in Escherichia coli JB525; see also Figure S5.
DISCUSSION
Structure-activity Relationships of the Honaucins
Anti-inflammatory and QS inhibition assay of the naturally-occurring honaucins and their synthetic analogs indicated that each of the functional groups comprising the honaucins is critical for the anti-inflammatory and QS inhibition activity, with the exception that the chlorine atom can be substituted with either bromine or iodine, but not fluorine, and retain high potency. Most modifications to the ring structure, such as the position and configuration of the substitutions on the lactone ring, as well as the absence of a conjugated double bond or halogenation in the carboxylic acid fragment, decreased activity in both assay systems (Table 2). Exceptions to these trends were 4′-bromohonaucin A (25) and 4′-iodohonaucin A (26) which both showed potent anti-inflammatory and QS inhibitory activities without appreciable toxicity. Their potency, compared to that of honaucin A (1), was increased nearly 30 times in the QS inhibition and 3–5 times in the anti-inflammatory assay. The correlation of biological activity with the ease of displacement of the halogen leaving group suggests that the activity of the honaucins may involve nucleophilic reactions at C-4′. However, inconsistent with this hypothesis, both 4-fluorocrotonic acid (13) and 4′-fluorohonaucin A (24) in which the fluorine atoms are not easily displaced, showed similar or more potent anti-inflammatory activities than 4-chlorocrotonic acid (7) or honaucin A (1), respectively. This suggests that honaucin inhibition of the innate immune response occurs by mechanisms other than, or in addition to, halogen atom nucleophilic displacement. 4′-Iodohonaucin A (26) was the most active compound among this series of honaucins. However, it was found to be quite labile, presumably through a facile displacement of the iodine atom, and therefore 4′-bromohonaucin A (25) may be the most promising candidate for further development.
Honaucin A (1) displayed much more potent anti-inflammatory and QS inhibitory activities than either of its active component fragments, (S)-3-hydroxy γ-butyrolactone (4) and 4-chlorocrotonic acid (7). These results suggest that a synergism is achieved when these two structural units are combined within a single molecule, and it is therefore conceivable that the honaucins have resulted through an evolutionary process that is conceptually similar to fragment-based drug design.
Honaucin A and Inhibition of Quorum Sensing
Intraspecies QS in Gram negative bacteria is most commonly regulated by AHLs (Galloway et al, 2011). Honaucin A is structurally similar to the AHLs, retaining both the γ-butyrolactone and a linear acyl side chain. However, the small structural alterations present in the honaucins appear to modulate their binding characteristics so as to act as antagonists, rather than inducers, of QS regulated phenotypes.
A handful of QS antagonists have been isolated from various marine organisms (Clark et al., 2008; Dobretsov et al., 2010; Givskov et al., 1996; Kwan et al., 2010; Kwan et al., 2011; Teasdale et al., 2009; Teasdale et al., 2010), and four such inhibitors have been previously isolated from marine cyanobacteria. However, the honaucins are chemically distinct from each of these. Lyngbyoic acid, isolated from Lyngbya cf. majuscula, modestlyinhibited QS in P. aeruginosa, reducing elastase and pyocyanin production (Kwan et al, 2011). The tumonoic acids obtained from Blennothrix cantharidosmum also showed modest activity, inhibiting bioluminescence production in V. harveyi with an IC50 of 62 μM (Clark et al., 2008). Malyngamide C and 8-epi-malyngamide C moderately inhibited LasR-based QS in P. aeruginosa. (Kwan et al., 2010). Finally, malyngolide which was obtained from Lyngbya majuscula, modestly inhibited (IC50= 110 μM) the production of violacein, a QS regulated phenotype in Chromobacterium violaceum (Dobretsov et al, 2010). In comparison, honaucins A–C are considerably more potent in their inhibition of V. harveyi regulated bioluminescence with IC50’s ranging from 5.6 to 17.6 μM. Furthermore, honaucin A inhibited the V. fischeri LuxI/R QS pathway in E. coli JB525 with an IC50 of 38.5 μM. Together, these results suggest that honaucin A inhibits AHL-mediated QS pathways, but further studies will be required to clarify the precise molecular mechanism of action of this new metabolite class.
Honaucin A and Inhibition of Eukaryotic Inflammation
Honaucin A and its brominated and iodinated analogs inhibited NO production in LPS stimulated macrophages with IC50s ranging from 0.8 to 4.0 μM. In addition to their potent biological activity and modest cytotoxicity, these molecules are small, structurally concise and synthetically tractable. These qualities present the honaucins as attractive potential anti-inflammatory drug leads.
There is considerable need to develop new anti-inflammatory treatments that have alternative molecular targets compared to the more traditional agents which target cyclooxygenase (COX) 1 and 2. Many patients are allergic to or suffer adverse side effects from COX inhibitors, limiting their treatment options (Farooque and Lee, 2009; Sánchez-Borges et al, 2004; Lanas, 2010). While the molecular target of the honaucins is yet to be identified, continuing efforts are underway to define the precise mechanism of action for these new modulators of inflammation.
Quorum Sensing Inhibition and Anti-inflammatory Activity
The honaucins and several of their synthetic analogs were potent inhibitors of both bacterial QS and NO production by LPS-stimulated macrophages. These two bioactivities may be mechanistically related. A scatter plot of these two activities of the honaucins displayed a positive correlation (Figure S5). Ours is not the first report of a biomolecule having dual involvement in QS and inflammation. Kravchencko et al. (2008) observed attenuated inflammation in activated bone marrow-derived macrophages in the presence of the P. aeruginosa QS signaling molecule N-(3-Oxododecanoyl)-L-homoserine lactone. This compound was found to exert anti-inflammatory effects by impairing the activation of NF-κB-dependent gene expression. However, not all accounts describe attenuation of the immune response; there have also been reports of immune activation by inhibiting the transcriptional activity of peroxisome proliferator activated receptor gamma (PPARγ) (Jahoor et al., 2008, Cooley et al., 2010, Teplitski et al., 2011). This discrepancy may be explained by a biphasic role for AHLs in immunomodulation in which they exert anti-inflammatory effects at low concentrations and stimulate inflammation at higher concentrations (Hughes and Sperandio, 2008).
Manoalide is another example of a natural product that is a QS inhibitor and possesses anti-inflammatory properties. The sesterpene metabolite, originally isolated from the Indo-Pacific sponge Luffariella variabilis (de Silva and Scheuer, 1980), was found to exert anti-inflammatory effects through an irreversible inhibition of PLA2 as well as antimicrobial activity through a strong inhibition of QS signaling. This latter activity was observed in both a promiscuous LuxR-regulated QS system cloned into E. coli and a P. aeruginosa-specific QS screen (Skindersoe et al., 2008). Thus, a commonality in the signaling pathways for prokaryotic QS and eukaryotic inflammation is potentially responsible for the pleiotropic effects of the honaucins and their analogues (Jahoor et al., 2008), a phenomenon described as ‘evolutionary molecular modeling’ (Wink, 2003).
SIGNIFICANCE
Three structurally novel metabolites, honaucins A–C (1–3), were isolated from the bloom-forming cyanobacterium Leptolyngbya crossbyana and show potent anti-inflammatory and QS inhibitory properties. Honaucin A (1) consists of two distinct structural units, (S)-3-hydroxy-γ-butyrolactone and 4-chlorocrotonic acid. In honaucin B (2), the lactone of honaucin A is opened to form the ethyl ester and the 4-chlorocrotonic acid is esterified to the C-4 hydroxy group rather than the C-3 hydroxy group as in honaucin A. Honaucin C (3) was found to be the methyl ester equivalent of honaucin B (2). These three metabolites inhibited LPS-stimulated NO production and repressed expression of pro-inflammatory cytokines in murine macrophages. Furthermore, all three compounds were effective at inhibiting QS signaling-dependent phenotypes in V. harveyi BB120 and E. coli JB525. Biological screening of synthetic analogs of honaucin A as halogen atom at the 4-position of the crotonoic acid subunit, were crucial for both anti-inflammatory and QS inhibitory properties. The analogs 4′-bromohonaucin A (25) and 4′-iodohonaucin A (26) were discovered to have more potent anti-inflammatory and QS inhibitory effects compared to the natural honaucins. Due to its stability and potent biological properties, 4′-bromohonaucin A (25) may represent a valuable lead for the development of drugs with anti-inflammatory and bacterial QS inhibitory properties.
EXPERIMENTAL PROCEDURES
General Experimental Procedures
Collection and Identification of Marine Cyanobacteria
The colonial cyanobacterium L. crossbyana (HI09-1) was collected in January 2009 by SCUBA from Hōnaunau reef, Hawai’i, at a depth of 20 m (GPS coordinates N19°42.338′, W155°91.292′). Samples were stored in 70% EtOH at −20 °C prior to extraction. The collected specimen was morphologically and phylogenetically identified in a previous study (Choi et al., 2010).
Extraction and Isolation
The cyanobacterial tissue was extracted repetitively with 2:1 CH2Cl2-CH3OH to yield 1.7 g of crude extract. A portion of the extract (1.4 g) was fractionated by silica gel VLC with a stepped gradient elution of hexanes, EtOAc, and MeOH. The fraction eluting with 40% hexanes in EtOAc was dried under reduced pressure, dissolved in hexanes and extracted with MeOH. The MeOH soluble material was fractionated using a Silica Sep-Pak column. The fraction eluting with 50% hexanes in EtOAc was subsequently subjected into RP-HPLC (Phenomenex Fusion RP 4 μ C18, 250 × 10 mm, 50% CH3CN/H2O at 3 mL/min) to give pure honaucin A (1, 5.4 mg, 0.39%) and honaucin B (2, 1.4 mg, 0.10%). The fraction eluting with 75% hexanes in EtOAc on Silica Sep-Pak chromatography was also subjected to RP-HPLC (Phenomenex Fusion RP 4 μ C18, 250 × 10 mm, 50% CH3CN/H2O at 3 mL/min) to give pure honaucin C (3, 1.1 mg, 0.08%).
Honaucin A (1): colorless oil; [α]D22 −80.5 (c 0.2, MeOH); UV (MeCN) λmax 205 nm (log ε 4.17); IR (neat) νmax 3010, 2955, 1784, 1724, 1316, 1270, 1167, 991 cm−1; 1H, 13C and 2D NMR data, see Table 1; HRESITOFMS m/z [M+H]+ 205.0262 (calcd for C8H10ClO4 205.0262).
Honaucin B (2): colorless oil; [α]D22 −8.0 (c 0.2, CH2Cl2); UV (MeCN) λmax 206 nm (log ε 4.33); IR (neat) νmax 3454, 2983, 1724, 1659, 1316, 1272, 1182, 1028 cm−1; 1H, 13C and 2D NMR data, see Table 1; HRESITOFMS m/z [M+Na]+ 273.0499 (calcd for C10H15ClNaO5 273.0500).
Honaucin C (3): colorless oil; [α]D22 −8.7 (c 0.2, CH2Cl2); UV (MeCN) λmax 206 nm (log ε 4.31); IR (neat) νmax 3451, 2982, 1724, 1659, 1316, 1272, 1182, 1029 cm−1; 1H and 13C NMR data, see Table 1; HRESITOFMSm/z [M+Na]+ 259.0346 (calcd for C9H13ClNa O5 259.0344).
Preparation of Synthetic Honaucin A and Its Analogs
See Supplemental Experimental Procedures for preparation and analytical data of synthetic honaucin A and its analogs.
Assay for the Detection of NO Production by Murine Macrophages
Measurement of NO production by murine macrophages stimulated with lipopolysaccharide, in the presence and absence of natural products and synthetic analogs, followed our previously published procedures (Villa et al., 2010), and is given in detail in the Supplemental Experimental Procedures.
Assessment of the Antioxidant Capacity of Honaucin A
See Supplemental Experimental Procedures for a detailed experimental description of assessment of the antioxidant capacity of honaucin A.
RNA Isolation and cDNA Synthesis
See Supplemental Experimental Procedures for a detailed experimental description of RNA isolation and cDNA synthesis.
Quantitative Real-time Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction of cDNA isolated from cells treated with honaucin A or other experimental conditions followed established protocols that are given in detail in the Supplemental Experimental Procedures.
Quorum Sensing Reporter Strains and Assays
Quorum sensing assays were run in two microbial systems, V. harveyi BB120 (Bassler et al., 1997), a wild-type, bioluminescent strain, and Escherichia coli JB525 (E. coli MT102 harboring the gfp plasmid pJBA132) which produces an unstable GFP in response to C6-C8 AHL autoinducers (Andersen et al., 2001). Detailed descriptions of the experimental protocols are given in the Supplemental Experimental Procedures.
Statistics
All experiments included three replicates. Data are presented as the mean ± standard deviation for the indicated number of technical replicates. Student’s t-test was used for the determination of statistical significance with p < 0.05 being considered significant. Prism software (Graphpad Software Inc., San Diego, CA) was used to generate figure graphs displaying SD.
Supplementary Material
Highlights.
Honaucins A–C were isolated from the cyanobacterium Leptolyngbya crossbyana.
Honaucins A–C displayed potent anti-inflammatory and QS inhibitory activities.
SAR study revealed crucial structural features for bioactivity of the honaucins.
4′-Bromohonaucin A is a potent modulator of inflammation and quorum sensing.
Acknowledgments
We gratefully acknowledge support of this research from UC San Diego and NIH NS053398. We thank the B. S. Moore laboratory (UCSD) for a sample of 4-fluorocrotonic acid.
Footnotes
ACCESSION NUMBER
The 16S rRNA gene sequence of L. crossbyana is available in the DDBJ/EMBL/GenBank databases under accession number GU111930.
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at doi:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen JB, Heydorn A, Hentzer M, Eberl L, Geisenberger O, Christensen BB, Molin S, Givskov M. gfp-Based N-acyl-homoserine-lactone sensor systems for detection of bacterial communication. Appl Environ Microb. 2001;67:575–585. doi: 10.1128/AEM.67.2.575-585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KP, Taylor KR, Jameson JM. Immunomodulation at epithelial sites by obesity and metabolic disease. Immunol Res. 2011 Dec 13; doi: 10.1007/s12026–011–8261–7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Choi H, Engene N, Smith JE, Preskitt LB, Gerwick WH. Crossbyanols AD, toxic brominated polyphenyl ethers from the Hawai’ian bloom-forming cyanobacterium Leptolyngbya crossbyana. J Nat Prod. 2010;73:517–522. doi: 10.1021/np900661g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BR, Engene N, Teasdale ME, Rowley DC, Matainaho T, Valeriote FA, Gerwick WH. Natural products chemistry and taxonomy of the marine cyanobacterium Blennothrix cantharidosmum. J Nat Prod. 2008;71:1530–1537. doi: 10.1021/np800088a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley MA, Whittall C, Rolph MS. Pseudomonas signal molecule 3-oxo-C12-homoserine lactone interferes with binding of rosiglitazone to human PPARgamma. Microbes Infect. 2010;12:231–237. doi: 10.1016/j.micinf.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Conaghan PG. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int. 2011 doi: 10.1007/s00296-011-2263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva ED, Scheuer PJ. Manoalide, an antibiotic sesterterpenoid from the marine sponge Luffariella variabilis. Tetrahedron Lett. 1980;21:1611–1614. [Google Scholar]
- Dobretsov S, Teplitski M, Alagely A, Gunasekera SP, Paul VJ. Malyngolide from the cyanobacterium Lyngbya majuscula interferes with quorum sensing circuitry. Environ Microbiol Rep. 2010;2:739–744. doi: 10.1111/j.1758-2229.2010.00169.x. [DOI] [PubMed] [Google Scholar]
- Farooque SP, Lee TH. Aspirin-sensitive respiratory disease. Annu Rev Physiol. 2009;71:465–487. doi: 10.1146/annurev.physiol.010908.163114. [DOI] [PubMed] [Google Scholar]
- Flachsmann F, Schellhaas K, Moya CE, Jacobs RS, Fenical W. Synthetic pseudopterosin analogues: A novel class of antiinflammatory drug candidates. Bioorg Med Chem. 2010;18:8324–8333. doi: 10.1016/j.bmc.2010.09.067. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Lilley BN, Bassler BL. A genetic analysis of the functions of LuxN: a two component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol Microbiol. 2000;35:139–149. doi: 10.1046/j.1365-2958.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- Galloway WRDJ, Hodgkinson JT, Bowden SD, Welch M, Spring DR. Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev. 2011;111:28–67. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- Gautam R, Jachak SM. Recent developments in anti-inflammatory natural products. Med Res Rev. 2009;29:767–820. doi: 10.1002/med.20156. [DOI] [PubMed] [Google Scholar]
- Gershon H, Shanks L, Gawiak DE. Antifungal activity of 4-substituted crotonic acid esters. J Med Chem. 1976;19:1969–1972. doi: 10.1021/jm00230a019. [DOI] [PubMed] [Google Scholar]
- Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg PD, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke JM, Bassler BL. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J Bacteriol. 2004;186:6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol. 2008;6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoor A, Patel R, Bryan A, Do C, Krier J, Watters C, Wahli W, Li G, Williams SC, Rumbaugh KP. Peroxisome prolieferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer. J Bacteriol. 2008;190:4408–4415. doi: 10.1128/JB.01444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC, Lehmann M, Meijler MM, Janda KD, Ulevitch RJ. Modulation of gene expression via disruption of NF-κB signaling by a bacterial small molecule. Science. 2008;321:259–263. doi: 10.1126/science.1156499. [DOI] [PubMed] [Google Scholar]
- Kwan JC, Meickle T, Ladwa D, Teplitski M, Paul V, Luesch H. Lyngbyoic acid, a “tagged” fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Mol BioSyst. 2011;7:1205–1216. doi: 10.1039/c0mb00180e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan JC, Teplitski M, Gunasekera SP, Paul VJ, Luesch H. Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscula. J Nat Prod. 2010;73:463–466. doi: 10.1021/np900614n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanas A. A review of the gastrointestinal safety data-a gastroenterologist’s perspective. Rheumatology. 2010;49(Suppl 2):ii3–10. doi: 10.1093/rheumatology/keq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery CA, Salzameda NT, Sawada D, Kaufmann GF, Janda KD. Medicinal chemistry as a conduit for the modulation of quorum sensing. J Med Chem. 2010;53:7467–7489. doi: 10.1021/jm901742e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai M, Nyholm SV, Castillo MG. The role of the immune system in the initiation and persistence of the Euprymna scolopes-Vibrio fischeri symbiosis. Semin Immunol. 2010;22:48–53. doi: 10.1016/j.smim.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam-Kia S, Werth VP. Prevention and treatment of systemic glucocorticoid side effects. Int J Dermatol. 2010;49:239–248. doi: 10.1111/j.1365-4632.2009.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle DG, Paul VJ. Production of secondary metabolites by filamentous tropical marine cyanobacteria: ecological functions of the compounds. J Phycol. 1999;35:1412–1421. [Google Scholar]
- Ni N, Choudhary G, Peng H, Li M, Chou HT, Lu CD, Gilbert ES, Wang B. Inhibition of quorum sensing in Vibrio harveyi by boronic acids. Chem Biol Drug Des. 2009a;74:51–56. doi: 10.1111/j.1747-0285.2009.00834.x. [DOI] [PubMed] [Google Scholar]
- Ni N, Li M, Wang J, Wang B. Inhibitors and antagonists of bacterial quorum sensing. Med Res Rev. 2009b;29:65–124. doi: 10.1002/med.20145. [DOI] [PubMed] [Google Scholar]
- Ogier JC, Calteau A, Forst S, Goodrich-Blair H, Roche D, Rouy Z, Suen G, Zumbihl R, Givaudan A, Tailliez P, Médigue C, Gaudriault S. Units of plasticity in bacterial genomes: new insight from the comparative genomics of two bacteria interacting with invertebrates, Photorhabdus and Xenorhabdus. BMC Genomics. 2010;11:568. doi: 10.1186/1471-2164-11-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas K, Winans S. A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes. Mol Microbiol. 2003;48:1059–1073. doi: 10.1046/j.1365-2958.2003.03488.x. [DOI] [PubMed] [Google Scholar]
- Prasad K, Chen KM, Repic O, Hardtmann GE. A highly stereoselective route to the four stereoisomers of a six-carbon synthon. Tetrahedron: Asymmetry. 1990;1:307–310. [Google Scholar]
- Rosenkranz G, Mancera O, Gatica J, Djerassi C. Steroids. IV α-Iodoketones A method for the conversion of allosteroids into Δ4–3-ketosteroids. J Am Chem Soc. 1950;72:4077–4080. [Google Scholar]
- Rumbaugh KP, Kaufmann GF. Exploitation of host signaling pathways by microbial quorum sensing signals. Curr Opin Microbiol. 2011 Dec 26;11 doi: 10.1016/j.mib.2011.12.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sánchez-Borges M, Capriles-Behrens E, Caballero-Fonseca F. Hypersensitivity to non-steroidal anti-inflammatory drugs in childhood. Pediatr Allergy Immunol. 2004;15:376–380. doi: 10.1111/j.1399-3038.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- Skindersoe ME, Ettinger-Epstein P, Rasmussen TB, Bjarnsholt T, de Nys R, Givskov M. Quorum sensing antagonism from marine organisms. Mar Biotechnol. 2008;10:56–63. doi: 10.1007/s10126-007-9036-y. [DOI] [PubMed] [Google Scholar]
- Smith JE, Kuwabara J, Flanaga K, duPlessis S, Coney J, Beets J, Takabayashi M, Barnes S, Turner J, Brown D, Griesemer BK, Stanton F. An unusual cyanobacterial bloom in Hawai’i. Coral Reefs. 2008;27:851. [Google Scholar]
- Smith RS, Kelly R, Iglewski BH, Phipps RP. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J Immunol. 2002a;169:2636–2642. doi: 10.4049/jimmunol.169.5.2636. [DOI] [PubMed] [Google Scholar]
- Smith RS, Harris SG, Phipps R, Iglewski B. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol. 2002b;184:1132–1139. doi: 10.1128/jb.184.4.1132-1139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swem LR, Swem DL, O’Loughlin CT, Gatmaitan R, Zhao B, Ulrich SM, Bassler BL. A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol Cell. 2009;35:143–153. doi: 10.1016/j.molcel.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swem LR, Swem DL, Wingreen NS, Bassler BL. Deducing receptor signaling parameters from in vivo analysis: LuxN/AI-1 quorum sensing in Vibrio harveyi. Cell. 2008;134:461–473. doi: 10.1016/j.cell.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale ME, Donovan KA, Forschner-Dancause SR, Rowley DC. Gram-positive marine bacteria as a potential resource for the discovery of quorum sensing inhibitors. Mar Biotechnol. 2010 doi: 10.1007/s10126-010-9334-7. [DOI] [PubMed] [Google Scholar]
- Teasdale ME, Liu J, Wallace J, Akhlaghi F, Rowley DC. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing- controlled phenotypes in gram-negative bacteria. Appl Environ Microb. 2009;75:567–572. doi: 10.1128/AEM.00632-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S-W, Schaffer JN, Tu KC, Mehta P, Lu W, Ong NP, Bassler BL, Wingreen NS. Active regulation of receptor ratios controls integration of quorum-sensing signals in Vibrio harveyi. Mol Syst Biol. 2011;7:491. doi: 10.1038/msb.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplitski M, Mathesius U, Rumbaugh KP. Perception and degradation of N-acyl homoserine lactone quorum sensing signals by mammalian and plant cells. Chem Rev. 2011;111:100–116. doi: 10.1021/cr100045m. [DOI] [PubMed] [Google Scholar]
- Terracciano S, Aquino M, Rodriquez M, Monti MC, Casapullo A, Riccio R, Gomez-Paloma L. Chemistry and biology of anti-inflammatory marine natural products: molecules interfering with cyclooxygenase, NF-κB and other unidentified targets. Curr Med Chem. 2006;13:1947–1969. doi: 10.2174/092986706777585095. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Kampoli AM, Papageorgiou N, Androulakis E, Antoniades C, Toutouzas K, Stefanadis C. Pathophysiology of atherosclerosis: the role of inflammation. Curr Pharm Des. 2011;17:4089–4110. doi: 10.2174/138161211798764843. [DOI] [PubMed] [Google Scholar]
- Tidgewell K, Clark BT, Gerwick WH. The natural products chemistry of cyanobacteria. In: Moore B, Crews P, editors. Comprehensive natural products chemistry. 2. Oxford, UK: Elsevier; 2010. pp. 141–188. [Google Scholar]
- Uchikawa O, Okukado N, Sakata T, Arase K, Terada K. Synthesis of (S)- and (R)-3-Hydroxy-4-butanolide and (2S,4S)-, (2R,4S)-, (2S,4R)-, and (2R,4R)-2-hydroxy-4-hydroxymethyl-4-butanolide and their satiety and hunger modulating activities. Bull Chem Soc Jpn. 1988;61:2025–2029. [Google Scholar]
- Villa FA, Gerwick L. Marine natural product drug discovery: leads for treatment of inflammation, cancer, infections, and neurological disorders. Immunopharmacol Immunotoxicol. 2010;32:228–237. doi: 10.3109/08923970903296136. [DOI] [PubMed] [Google Scholar]
- Villa FA, Lieske K, Gerwick L. Selective MyD88-dependent pathway inhibition by the cyanobacterial natural product malyngamide F acetate. Eur J Pharmacol. 2010;629:140–146. doi: 10.1016/j.ejphar.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochem. 2003;64:3–19. doi: 10.1016/s0031-9422(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Zhang R, Pappas T, Brace J, Miller P, Oulmassov T, Molyneaux J, Anderson J, Bashkin J, Winans S, Joachimiak A. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature. 2002;417:971–974. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.