Abstract
In the current study, soils of Tang-e Douzan mine, located in Isfahan, Iran, were collected and analyzed for soluble, exchangeable, and total amounts of Pb, Zn, Cd, Ca, and Mg. The maximum Pb, Zn, Cd, Ca, and Mg concentrations in soils were 2500, 1100, 59, 43,800, and 1320 mg/kg for total metals, 86, 83, 6.3, 4650, and 48 mg/kg for their exchangeable fractions, and 59, 3.7, 0.53, 430, and 6.4 mg/kg for their soluble fractions, respectively. All specimens collected, including 69 plant species, were analyzed for Pb, Zn, and Cd. Moreover, their phytoremediation potential was investigated by calculating bioconcentration factors (BCF), translocation factors (TF), and extraction factors (EF) for each heavy metal. Analysis of the leaves for heavy metals showed no metal hyperaccumulation. The highest shoot concentrations of Pb (298 mg/kg) and Zn (740 mg/kg) were found in Roemeria hybrida subsp. dodecandra and Cd (43 mg/kg) in Chenopodium foliosum. Plants having BCFs and TFs > 1 are capable of phytoextraction. Among the analyzed species, four had both TFs and BCFs > 1 for Zn, 13 for Cd, and none for Pb. R. hybrida, Bromus squarrosus, Descurainia sophia, and Poa bulbosa seem to be the best choices for phytoextraction of Zn. Aegilops columnaris, Allium ampeloprasum subsp. iranicum, B. squarrosus, and Cousinia piptocephala are the best choices for phytoextraction of Cd. Plants with BCF > 1 and TF < 1, including Cerastium dichotomum and Muscari neglectum for Pb, Ceratocephala falcata, M. neglectum, Ornithogalum orthophyllum, and Ranunculus arvensis for Zn and C. falcata, M. neglectum, O. orthophyllum, and R. hybrida subsp. dodecandra for Cd, are proposed to be the most efficient species for metal phytostabilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term heavy metal (HM) refers to any element whose density is more than 5 (Lozet and Mathieu 1991; Adriano 2001) or 6 g/cm3 (Davies 1987; Thornton 1995). Heavy metals are a group of pollutants that are currently of much environmental concern due to their toxicity to a wide range of organisms. The high concentration of heavy metals in soil is not only caused by natural processes as a result of weathering of rock minerals, but also by anthropogenic activities, such as mining, metal ores smelting, gas exhaust, energy/fuel production, electroplating, industrial emission, and the use of fertilizers and insecticides (Baker 1987; Alloway 1994; Garbisu and Alkorta 2003). In recent decades, the annual global release of heavy metals reached 22,000, 783,000, 939,000, and 1,350,000 t (metric tons) for cadmium (Cd), lead (Pb), copper (Cu), and zinc (Zn), respectively (Singh et al. 2003).
In recent decades, interest in ecological threats of heavy metals led to in-depth research on new economical remediation technologies based on plants. Conventional methods employed for remediation of contaminated soils, including chemical, physical, and microbiological methods, are expensive and time-consuming; they can also release secondary wastes and put workers at risk (Wenzel et al. 1999; Lombi et al. 2000; Fitz and Wenzel 2002; Danh et al. 2009). An encouraging and eco-friendly approach is phytoremediation technology wherein plants are utilized to extract, sequester, and/or detoxify contaminants to prevent their dissipation by groundwater or run-off or impede their accumulation via food webs (McGrath et al. 2002; Suresh and Ravishanker 2004; Arthur et al. 2005). It has been reported that phytoremediation is an efficient, non-intrusive, cheap, and esthetically pleasant technology for remediation of contaminated natural environments (Alkorta and Garbisu 2001; Weber et al. 2001; Garbisu et al. 2002).
Plants exhibit three strategies in dealing with high concentrations of heavy metals: exclusion, indication, and accumulation. Excluders are those plants that do not let metal enter their aerial parts and, hence, have a comparatively low metal concentration in the shoot over a wide range of soil metal concentrations by keeping metals in their roots. Indicator plants accumulate metals in their shoots and express some measure of the soil bioavailable metal content. Finally, accumulators translocate and concentrate metals in their aerial parts up to the levels higher than those of the soil (Baker 1981; Raskin et al. 1994; Cunningham 1995).
Over 500 plant species, named hyperaccumulators, concentrate high amounts of trace elements (Reeves and Baker 2000). Hyperaccumulators have been defined as plants that can accumulate more than 10,000 mg/kg of Mn or Zn, 1000 mg/kg of Pb, Ni, Cu, Co, As, or Se, and 100 mg/kg of Cd (Baker and Brooks 1989; Robinson et al. 2006) in their foliage dry matter. Van der Ent et al. (2013) defined hyperaccumulators as plants that can accumulate more than 10,000 mg/kg of Mn, 3000 mg/kg of Zn, 1000 mg/kg of As, Ni, and Pb, 300 mg/kg of Cr, Cu, and Co, and 100 mg/kg of Tl, Cd, and Se in their shoot dry matter. For different elements, the approximate numbers of hyperaccumulator plants are 450 for Ni, 32 for Cu, 30 for Co, 20 for Se, 14 for Pb, 12 for Mn, 12 for Zn, 5 for As, 2 for Cd, and 2 for Tl (Van der Ent et al. 2013). As put forth by some researchers (Arthur et al. 2005), there is a need for better understanding of potential mechanisms in the removal of contaminants from the root zone which is helpful in expanding the application of phytoremediation to clean up other contaminated sites.
Phytoremediation of contaminated soils is achievable by various processes such as rhizofiltration, phytostabilization or phytoimmobilization, phytodegradation, phytotransformation, phytovolatilization, phytostimulation, and phytoextraction (Schwitzguebel 2000; Ali et al. 2013). Among these strategies, phytoextraction (phytoaccumulation), which is a plant’s capability to absorb inorganic (mainly metal) contaminants from soil, is the most prevalent remediation technology (McCutcheon and Schnoor 2003). Phytostabilization is defined as the ability of certain plants to reduce the availability of toxic metals in soil (Gwozdz and Kopyra 2003). Such a process could reduce the danger of the pollutant, but it could not remove toxic metals from the soil (Li et al. 2000).
Phytoremediation efficiency can be measured by calculating bioconcentration factor (BCF) and translocation factor (TF). BCF, defined as the ratio of metal concentration in the roots to that in the soil, could be employed to estimate the efficiency of a plant in absorbing metals from soils (Zhuang et al. 2007; Ladislas et al. 2012; Mahdavian et al. 2017). According to Yoon et al. (2006), plants with BCF and TF greater than 1 have the potential to be used for phytoextraction, while plants with a BCF more than 1 and a TF less than 1 could be useful in phytostabilization. Plants showing TF and BCF values less than 1 are not suitable for phytoextraction (Fitz and Wenzel 2002).
The flora of Iran is made up of about 8000 plant species from 150 families. About 1727 of these species are endemic to Iran (Jalili and Jamzad 1999). Iran has several natural metalliferous (metal-containing soils) and anthropogenic metal-contaminated soils, but not much data is available on their flora and the concentration of trace elements in plants (Ghaderian and Baker 2007). Metal exposure through millennia has driven the evolution of metal resistance in metallophytes (plants that are specifically adapted to and flourish in heavy metal-rich soils) under local environmental conditions (Ernst 2000). Thus, there are possibilities to find locally adapted species in Tang-e Douzan Pb-Zn mine, with beneficial properties for the phytoremediation of metal-polluted soils.
The aims of the present study are as follows: (1) identification of plant species growing on heavily polluted soils at Tang-e Douzan Pb-Zn mine, (2) determination of Pb, Zn, and Cd concentrations in the soils and plants, and (3) evaluation of the usefulness of these plants to remediate metal-contaminated soils.
Materials and methods
Site description
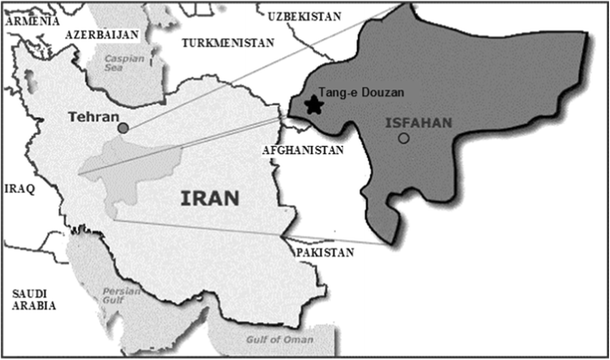
Tang-e Douzan Zn-Pb mining area is located near Fereydoonshahr, 175 km west of Isfahan, central Iran (Fig. 1), in a mountainous area at 2800–2850 m asl, 49° 57′ 7″ E and 33° 2′ 39″ N. Mean annual rainfall is 546.4 mm, about 41% in winter, 31% in autumn, and 24% in the spring. Maximum and minimum temperatures, 34.6 and − 25 °C, in summer and winter, respectively, were recorded at the nearest weather station from January 2010 to September 2016. The main oxide and carbonate minerals in this deposit are hemimorphite, smithsonite, and cerusite; galena, sphalerite, and pyrite are also the main sulfide minerals. In this open mining area, the surface soils naturally include high concentrations of Zn, Pb, and Cd. In this study, soil and plants were collected from four different sites around the mine: the eastern (site 1), western (site 2), southern (site 3), and northern (site 4) sides of the excavated location.
Plant and soil sampling and analysis
From May 2015 to August 2016, plants growing on four sites of the Tang-e Douzan Pb-Zn mine were collected for identification, metal analysis, and preservation of reference samples. Samples of the soils were taken near plant roots (0–20 cm depth). In order to analyze Pb, Zn, and Cd in soil, samples were air-dried and passed through a < 2-mm sieve. Five grams of each sample was ground, sieved with 80-mesh screen, and oven-dried at 70 °C. Then, a 0.5-g sub-sample was digested with 10 ml of a mixture of 3:1 HCl/HNO3 acids. Tubes were kept at room temperature for a night and then put in a water bath at 80 °C for 2 h. After they were cooled, each digest was filtered through a moisturized Whatman No. 40 filter paper into a 10-ml volumetric flask. Afterwards, flasks were made up to volume with distilled water. Analyses of Pb, Zn, Cd, Mg, and Ca were done using an atomic absorption spectrophotometer (AAS; Shimadzu AA-6200). Exchangeable elements were determined based on the ammonium acetate method (Sparks 1996) by extracting with a 1:4 ratio of soil to extraction solution (1 mol/L NH4OAc, pH 7.0). The suspension was shaken in an end-over-end shaker at 20 °C for 2 h. Next, the soil suspension was left to stand for 5 min and then filtered into a clean test tube. Later on, the filtrate was acidified with HNO3 to 0.2% to analyze the above metals by AAS. To analyze the water-soluble metals, 1:10 proportion of soil and distilled water was mixed and then shaken with a rotary shaker for 24 h. Then, the solution was centrifuged at 3000 rpm and the supernatant was filtered. Finally, AAS was used to measure the concentration of Pb, Zn, Cd, Mg, and Ca in the filtered solution.
The plant samples were washed twice with distilled water. The plant shoots and roots (if present) were separated and oven-dried to constant weight at 70 °C. It should be noted that root sampling was not performed for some species due to a possible threat to the ecosystem. The oven-dried plant materials were ground so that homogenous samples were obtained; subsamples of 0.1 g dry weight were digested in a mixture of HNO3 (65%), HCl (37%), and H2O2 (30%) (6:3:1, v/v/v) and heated at 120 °C for 1 h. After the digests were cooled, they were made up to 10 ml with distilled water. Finally, the solutions were analyzed for Pb, Zn, and Cd (Shimadzu AA-6200). Soil pH was measured by glass electrode using a suspension of soil (10 g) and distilled water (30 ml).
Results
Concentrations of total, exchangeable, and soluble Pb, Zn, Cd, Ca, and Mg, together with the Mg/Ca ratio, in the sampled soils are shown in Table 1. Concentrations of Pb, Zn, Cd, and Ca in these soils are elevated, but Mg and Mg/Ca ratio is low in comparison with ultramafic (serpentine) soils (Reeves and Baker 2000; Reeves 1992). As shown in Table 1, variable total metal concentrations were observed in the soil samples, ranging from 40 to 2500 mg/kg Pb, 8.6 to 1100 mg/kg Zn, 1.9 to 60 mg/kg Cd, 4600 to 43,800 mg/kg Ca, and 890 to 1320 mg/kg Mg.
The exchangeable fraction of elements in the soil ranged from 1.8 to 86 mg/kg Pb, 1.4 to 83 mg/kg Zn, 0.4 to 6.3 mg/kg Cd, 3280 to 4650 mg/kg Ca, and 26 to 49 mg/kg Mg (Table 1). Soluble metal concentrations were 1.6–59 mg/kg for Pb, 0.001–3.7 mg/kg for Zn, 0.22–0.53 mg/kg for Cd, 227–430 mg/kg for Ca, and 1.6–6.4 mg/kg for Mg (Table 1). The Mg/Ca ratio of total metal concentrations was as low as 0.02 to 0.8. The pH of all soils was slightly basic, ranging from 7.85 to 8.33 (Table 1).
In this study, 69 vascular plant species were taken from different sites of the Tang-e Douzan mining area, from 60 genera and 21 families (Table 2). The main represented families were as follows: Asteraceae (16), Brassicaceae (6), Lamiaceae (6), Liliaceae (6), and Poaceae (6). Concentrations of Pb, Zn, and Cd in the shoots of all (and roots of some) the plants are shown in Table 2. Shoot metal concentrations varied from 14 mg/kg in Tanacetum polycephalum to 298 mg/kg in Roemeria hybrida for Pb, 47 mg/kg in Euphorbia cheiradenia to 740 mg/kg in R. hybrida for Zn, and 2 mg/kg in Stachys lavandulifolia to 43 mg/kg in Chenopodium foliosum for Cd.
Assessments of Pb concentration in the roots of some plants, where sampling was possible, were conducted and ranged from 14 to 1030 mg/kg Pb in Tulipa systola and Cerastium dichotomum, 66 to 2040 mg/kg Zn in Allium longivaginatum and Ornithogalum orthophyllum, and 1.8 to 46 mg/kg Cd in Euphorbia condylocarpa and Muscari neglectum, respectively (Table 2).
Discussion
This is the first article on the concentrations of Pb, Zn, and Cd in soils and plant species from the Tang-e Douzan mining area, Isfahan, Iran. According to Kabata-Pendias (2011), the overall mean values of total Pb, Zn, and Cd content of soils are estimated to be 27, 70, and 0.41 mg/kg, respectively. These values for Earth’s crust are 15, 70, and 0.1 mg/kg. The mean values of total soil Pb, Zn, and Cd contents of the 4 sampling sites (Table 1) exceeded these values (184–1480 mg/kg Pb; 395–976 mg/kg Zn, and 4.7–29 mg/kg Cd); therefore, soils of the sampling sites are contaminated by these heavy metals as compared to normal soils. This situation is caused by weathering of parent rocks in Tang-e Douzan mining area. This contamination has led to the lack of vegetation at some locations in the area.
A considerable fraction of total metals in the soil is insoluble and therefore is not instantly bioavailable. However, plants could have direct access to the soluble and exchangeable forms of heavy metals (Lorenz et al. 1997; Pollard et al. 2002).
The amounts of exchangeable Pb and Zn in the soil were limited to 0.3 and 5 mg/kg, respectively; the appraisal of hazard and remediation needs is required over these values (Pruess 1994; Ghaderian et al. 2007). Austrian Standards give the threshold values of 0.03, 1.8, and 0.003 for groundwater Pb, Zn, and Cd concentrations, respectively (BGBl. 504 1991). In the current research, the highest concentrations of exchangeable metals were 86 mg/kg Pb at site 2, 83 mg/kg Zn at site 4, and 6.3 mg/kg Cd at site 1. For soluble fractions, maximum values were 59 mg/kg at site 3, 3.7 mg/kg at site 1, and 0.53 mg/kg at site 3 for Pb, Zn, and Cd, respectively. Thus, large amounts of Pb, Zn, and Cd in the exchangeable and soluble fractions in these sites suggest high soil toxicity. These values show considerable increase in bioavailability of metals, but the toxicity of a specific metal to an organism could not easily be estimated just by the exchangeable or soluble fractions of that element concentration (Otero et al. 2012; Mahdavian et al. 2017). It is important to consider the role of other factors. Differences in the availability and uptake of metal ions may result from their chemical forms, soil pH variations, major element concentrations in soil (e.g., Ca and Mg), and some physical properties, for instance, soil evaporation and porosity together with the local rainfall (Thornton 1999; Van der Ent et al. 2013).
The bioavailabilities of most of the heavy metals in alkaline soils are low in comparison with acidic soils (Wong 2003; Mahdavian et al. 2017). The pH of the sampled soils in the current study was moderately alkaline (Table 1).
Kim et al. (2002) previously showed the protective effects of Ca and Mg on the uptake and toxicity of Pb and Cd in rice (Oryza sativa). They stated that molar ratios of Mg or Ca to Cd or Pb higher than 10:1 were essential for a 50% decrease in uptake of Cd and Pb, as compared to the control treatment. The highest total concentrations of Ca, Mg, Pb, and Cd in soil samples were 43,800, 1320, 2500, and 60 mg/kg, respectively. Maximum values for exchangeable fractions were 4650 for Ca, 49 for Mg, 86 for Pb, and 6.3 for Cd. These amounts are far beyond the 10:1 ratio, particularly in the case of Ca (290 for Ca:Pb, 2071 for Ca:Cd, 5 for Mg:Pb, and 36 for Mg:Cd). Thus, it can be concluded that high concentrations of Mg and especially Ca can reduce the uptake and toxicity of Pb and Cd to plants in Tang-e Douzan area.
Heavy metal uptake from the rhizosphere by plants occurs via passive transport by water mass flow into the roots or an active transport through the plasma membrane of root cells. Some plants may take up elements at a larger concentration than in the surrounding soil and water (Kim et al. 2003). It is expected that the 69 plant species collected from Tang-e Douzan Pb-Zn mine may take up high amounts of metals and show high metal tolerances.
Concentrations of 1 mg/kg Pb, 50 mg/kg Zn, and 0.05 mg/kg Cd are considered as the international standard reference for plants (Van der Ent et al. 2013). In this study, Pb concentrations in plant shoots ranged from 14 to 298 mg/kg with the maximum value in R. hybrida. As a result, no species had a Pb concentration more than 1000 mg/kg in its shoots, which is the notional criterion for hyperaccumulation of Pb (Baker and Brooks 1989; Van der Ent et al. 2013). Additionally, in roots of 10 species (out of 16 analyzed sampled ones), the concentrations of Pb were greater than those of shoots, with TFs ranging from 0.08 to 0.93 in C. dichotomum and A. longivaginatum, respectively (Table 3). This indicates little Pb root to shoot translocation and its immobilization in the roots. On the contrary, T. systola and C. falcata have TFs of 2.85 and 2.79, indicating high Pb root to shoot translocation. Concentrations of Pb in the plants ranged from 14 to 1030 mg/kg in roots, with the highest value in C. dichotomum roots. To date, 14 Pb hyperaccumulators have been reported (Van der Ent et al. 2013); thus, hyperaccumulation of Pb does not occur in most plants grown on Pb-contaminated soils (Reeves 1988; Reeves et al. 2001). Many reports are on the concentrations of Pb in plants which grow on contaminated environments. For instance, root Pb concentrations in the range of non-detectable to 1800 mg/kg were reported by Pitchtel et al. (2000). Furthermore, Stoltz and Greger (2002) showed Pb concentrations in a range of 3.4 to 920 mg/kg in the roots of various plant species growing on mine tailings. Yoon et al. (2006) revealed concentrations of Pb in aerial plant parts from non-detectable to about 500 mg/kg. The range of Pb concentrations between 8 mg/kg in Acantholimon aspadanum and 740 mg/kg in Pinus eldarica was also reported by Ghaderian et al. (2007).
As an essential micronutrient, Zn in plants is usually found at 10 to 200 mg/kg concentrations (Ghaderian and Ghotbi Ravandi 2012). In the present study, Zn concentrations in the plant shoots and roots had the ranges of 47–740 mg/kg and 66–2040 mg/kg, respectively. Its highest concentrations were found in R. hybrida shoots and of O. orthophyllum roots. Root Zn concentrations were more than those of shoots except for A. longivaginatum, Tragopogon graminifolius, Astragalus sp., and Scorzonera phaeopappa.
There are many reports on Zn concentrations in plants grown in contaminated environments. For example, ranges of 68 to 1630 mg/kg and 17 to 453 mg/kg Zn were reported by Stoltz and Greger (2002) and Yoon et al. (2006), respectively. Both studies’ ranges reported, however, were less than the observed amount in this study. In contrast, Zn concentrations (up to 7600 mg/kg) higher than those observed in the current study were reported by Shu et al. (2002).
Cd is regarded as one of the most eco-toxic metals, having negative effects on all biological processes of humans, animals, and plants. It shows a great potential to adversely affect food quality and the environment (Kabata-Pendias 2011). To date, 2 Cd hyperaccumulators have been reported (Van der Ent et al. 2013). In the current study, Cd concentration in plant shoots was more than the international standard amount for normal plants (0.05 mg/kg) and ranged from 2 to 43 mg/kg with the maximum value in C. foliosum. Thus, none of the plant species accumulated Cd in their shoots more than 100 mg/kg, the notional threshold for Cd hyperaccumulation (Baker and Brooks 1989; Van der Ent et al. 2013).
Cd concentrations in roots of sampled plants were in the range of 1.7 to 46 mg/kg with maximum values in the roots of M. neglectum. Similar studies by Stoltz and Greger (2002) reported Cd concentrations of 0.1 to 12.5 mg/kg. Rio et al. (2002) also reported concentrations of Cd ranging from undetectable to 9.7 mg/kg in analyzed plants. Moreno-Jimenez et al. (2009) showed Cd concentrations from undetectable to 22.04 mg/kg in shoots of plants. All these reports are at a range lower than that found in biomass of plants in this study.
Plants used for phytoremediation to impede the distribution of pollutants via wind, run-off or groundwater (phytostabilization), or to take up and remove contaminants from soil/water environments by accumulating them into shoot biomass (phytoextraction), must have certain characteristics. Plant species which grow fast, yield large biomass amounts, and take up high metal concentrations out of the soil, are encouraging candidates for phytoremediation (Anawar et al. 2006). Moreover, BCF and TF values determine which plants should be selected for phytoremediation purposes (Wu et al. 2011). According to Yoon et al. (2006), plants exhibiting BCF > 1 and a TF < 1 have the capacity to be used for phytostabilization while plants with TF and BCF values > 1 could be useful for phytoextraction. Some authors (Mattina et al. 2003; Ha et al. 2011) defined BCF as the ratio of metal concentration in shoot to that in the soil, whereas others assumed it as root to soil concentration of metal (Yoon et al. 2006; Mahdavian et al. 2017).
In this study, the second definition of BCF was applied and extraction factor (EF) was assumed for shoot to soil metal concentration.
According to Yoon et al. (2006) and Mahdavian et al. (2017):
and
And if we assume
Regarding
So,
where Cshoot and Croot are the concentrations of the metal in the plant shoot and root, respectively, and Csoil is the concentration of the same metal in the soil. Therefore, EF can reflect both BCF (the ability of plant to concentrate metal from soil to its root) and TF (translocation of metal from root to shoot) and can be an appropriate indicator to explain phytoextraction potential of a plant. For example, a plant may have a BCF < 1, but with a very high TF, it can compensate for the shortage of BCF, and hence, the plant can accumulate metal in its shoots in concentrations higher than that in the soil just like a plant with both BCF and TF > 1. Erysimum crassicaule with TF of 2.07 and BCF of 0.53 showed such a situation and had EF of 1.1 for Cd and accumulated it in shoots (7.1 mg/kg) more than soil (6.5 mg/kg) (Tables 2 and 3). O. orthophyllum and R. hybrida subsp. dodecandra showed a similar situation for Cd and Zn with TF < 1 and BCF > 1, but EF > 1 and may show potential for phytoextraction.
According to the data in Tables 3 and 4, plant species that could be suitable for Pb, Zn, and Cd phytostabilization were C. dichotomum for Pb, M. neglectum for all three metals, C. falcata for Zn and Cd, O. orthophyllum for Zn, and R. hybrida subsp. dodecandra for Cd. M. neglectum was the most appropriate sampled plant for phytostabilization of Zn and Cd and O. orthophyllum for Zn.
The best choices for phytoextraction of Zn were Bromus squarrosus, Poa bulbosa, and Descurainia sophia. Based on the results, no candidate for phytoextraction of Pb was observed, whereas B. squarrosus, Aegilops columnaris, Allium ampeloprasum subsp. iranicum, A. longivaginatum, Alyssum lanigerum, C. foliosum, Cousinia piptocephala, P. bulbosa, Orobanche aegyptiaca, E. cheiradenia, and D. sophia were the best choice for phytoextraction of Cd.
Bromus squarrosus, with maximum EF of 3.45, is regarded as the most encouraging species for phytoextraction of Zn and Cd contaminated sites. The use of hyperaccumulators has been tested for phytoextraction of heavy metals, which accumulate more metals but produce relatively less aboveground plant biomass. Another way is to use other plants, like the above-mentioned plants, that accumulate less heavy metal concentration but produce more aboveground plant biomass so that the overall metal content is comparable to that of hyperaccumulators (Robinson et al. 1998; Tlustoš et al. 2006).
Conclusions
Plants on Pb, Zn, and Cd contaminated soils of the Tang-e Douzan mine area were collected and identified. Afterwards, the concentrations of these metals in the plants and soil were determined. No single species (out of 69 collected) was identified as a hyperaccumulator. Only the species with both BCFs and TFs > 1, and EFs > 1, have the potential for phytoextraction. Among all the species screened, 4 plants had EF > 1 for Zn and 13 plants for Cd, but no plant showed EF > 1 for Pb. Thus, R. hybrida, B. squarrosus, D. sophia, and P. bulbosa could be the best choices for phytoextraction of Zn and B. squarrosus, A. columnaris, A. ampeloprasum subsp. iranicum, and C. piptocephala for phytoextraction of Cd. In addition, R. hybrida, Ranunculus arvensis, and Fritillaria imperialis with maximum shoot Pb concentrations (298, 247, and 244 mg/kg, respectively) seem to be the best choices for phytoextraction of Pb. Plants with BCF > 1 and TF < 1, including C. dichotomum and M. neglectum for Pb, C. falcata, M. neglectum, O. orthophyllum, and R. arvensis for Zn and C. falcata, M. neglectum, O. orthophyllum, and R. hybrida subsp. dodecandra for Cd, are suggested as the most effective species for phytostabilization of contaminated soils. The tolerance, metal accumulation, and phytoremediation potentials of these plants in controlled experimental condition and/or other contaminated soils with low Ca concentration could be considered as areas for further study.
References
Adriano DC (2001) Trace elements in terrestrial environments. Biochemistry, Alburry, Australia. Springer, New York, pp 1–16. https://doi.org/10.1007/978-0-387-21510-5
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals-concepts and applications. Chemosphere 91(7):869–881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Alkorta I, Garbisu C (2001) Phytoremediation of organic contaminants in soils. Bioresour Technol 79(3):273–276. https://doi.org/10.1016/S0960-8524(01)00016-5
Alloway BJ (1994) Toxic metals in soil-plant systems. Wiley, Chichester
Anawar HM, Garcia-Sanchez A, Murciego A, Buyolo T (2006) Exposure and bioavailability of arsenic in contaminated soils from the La Parrilla mine, Spain. Environ Geol 50(2):170–179. https://doi.org/10.1007/s00254-006-0196-2
Arthur EL, Rice PJ, Rice PJ, Anderson TA, Baladi SM, Henderson KLD, Coats JR (2005) Phytoremediation-an overview. Crit Rev Plant Sci 24(2):109–122. https://doi.org/10.1080/07352680590952496
Baker AJM (1981) Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutr 3(1-4):643–654. https://doi.org/10.1080/01904168109362867
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements-a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
BGBl. 504 (1991) Verordnungdes Bundesministers fur Landund Forstwirtschaft betreffend Schwellenwerte fur Grundwasserinhaltsstoffe (Grundwasserschwellenwertverordnung-GSwV)
Cunningham SD (1995) In proceedings/abstracts of the fourteenth annual symposium, current topics in plant biochemistry, physiology, and molecular biology columbia, April 19–22, pp 47–48
Danh LT, Truong P, Mammucari R, Tran T, Foster N (2009) Vetiver grass, Vetiveria zizanioides: a choice plant for phytoremediation of heavy metals and organic wastes. Int J Phytorem 11(8):664–691. https://doi.org/10.1080/15226510902787302
Davies BE (1987) Consequences of environmental contamination by lead mining in Wales. Hydrobiologia 149(1):213–220. https://doi.org/10.1007/BF00048662
Ernst WHO (2000) Evolution of metal hyperaccumulation and phytoremediation hype. New Phytol 146(3):357–358. https://doi.org/10.1046/j.1469-8137.2000.00669.x
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil-rhizosphere-plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99(3):259–278. https://doi.org/10.1016/S0168-1656(02)00218-3
Garbisu C, Alkorta I (2003) Basic concepts on heavy metal soil bioremediation. Eur J Miner Process Environ Prot 3:58–66
Garbisu C, Hernandez-Allica J, Barrutia O, Alkorta I, Becerril JM (2002) Phytoremediation: a technology using green plants to remove contaminants from polluted areas. Rev Environ Health 17:75–90
Ghaderian SM, Baker AJM (2007) Geobotanical and biogeochemical reconnaissance of the ultramafics of Central Iran. J Geochem Explor 92(1):34–42. https://doi.org/10.1016/j.gexplo.2006.06.002
Ghaderian SM, Ghotbi Ravandi AA (2012) Accumulation of copper and other heavy metals by plants growing on Sarcheshmeh copper mining area, Iran. J Geochem Explor 123:25–32. https://doi.org/10.1016/j.gexplo.2012.06.022
Ghaderian SM, Hemmat GR, Reeves RD, Baker AJM (2007) Accumulation of lead and zinc by plants colonizing a metal mining area in central Iran. J Appl Bot Food Qual 81:145–150
Gwozdz EA, Kopyra M (2003) Plant cell responses to heavy metals-biotechnological aspects. Biotechnologia 3:107–123
Ha NTH, Sakakibara M, Sano S, Nhuan MT (2011) Uptake of metals and metalloids by plants growing in a lead-zinc mine area, northern Vietnam. J Hazard Mater 186(2-3):1384–1391. https://doi.org/10.1016/j.jhazmat.2010.12.020
Jalili A, Jamzad Z (1999) Red data book of Iran. RIFR, Tehran
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Kim Y, Yang Y, Lee Y (2002) Pb and Cd uptake in rice roots. Physiol Plant 116(3):368–372. https://doi.org/10.1034/j.1399-3054.2002.1160312.x
Kim IS, Kang HK, Johnson-Green P, Lee EJ (2003) Investigation of heavy metal accumulation in Polygonum thunbergii for phytoextraction. Environ Pollut 126(2):235–243. https://doi.org/10.1016/S0269-7491(03)00190-8
Ladislas S, El-Mufleh A, Gérente C, Chazarenc F, Andrès Y, Béchet B (2012) Potential of aquatic macrophytes as bioindicators of heavy metal pollution in urban stormwater runoff. Water Air Soil Pollut 223(2):877–888. https://doi.org/10.1007/s11270-011-0909-3
Li YM, Chaney RL, Angle JS, Baker AJM (2000) Phytoremediation of heavy metal contaminated soils. Environ Sci Poll Con Ser 22:837–857
Lombi E, Sletten RS, Wenzel WW (2000) Sequentially extracted arsenic from different size fractions of contaminated soil. Water Air Soil Pollut 124(3/4):319–332. https://doi.org/10.1023/A:1005230628958
Lorenz SE, Hamon RE, Holm PE, Domingues HC, Sequeria EM (1997) Cadmium and zinc in plants and H2O-extracts from contaminated soils. Plant Soil 189(1):21–23. https://doi.org/10.1023/A:1004214923372
Lozet J, Mathieu C (1991) Dictionary of soil science, 2nd edn. A. A. Balkema, Rotterdam
Mahdavian K, Ghaderian SM, Torkzadeh-Mahani M (2017) Accumulation and phytoremediation of Pb, Zn, and ag by plants growing on Koshk lead-zinc mining area, Iran. J Soils Sediments 17(5):1310–1320. https://doi.org/10.1007/s11368-015-1260-x
Mattina MI, Lannucci-Berger W, Musante C, White JC (2003) Concurrent plant uptake of heavy metals and persistent organic pollutants from soil. Environ Pollut 124(3):375–378. https://doi.org/10.1016/S0269-7491(03)00060-5
McCutcheon SC, Schnoor JL (2003) Phytoremediation: transformation and control of contaminants. Wiley, Hoboken. https://doi.org/10.1002/047127304X
McGrath SP, Zhao FJ, Lombi E (2002) Phytoremediation of metals, metalloids, and radionuclides. Adv Agron 75:1–56. https://doi.org/10.1016/S0065-2113(02)75002-5
Moreno-Jimenez E, Penalosa JM, Manzano R, Carpena-Ruuiz RO, Gamarra R, Esteban E (2009) Heavy metals distribution in soils surrounding an abandoned mine in NWMadrid (Spain) and their transference to wild flora. J Hazard Mater 162(2-3):854–859. https://doi.org/10.1016/j.jhazmat.2008.05.109
Otero XL, Alvarez E, Fernandez-Sanjurjo MJ, Macias F (2012) Micronutrients and toxic trace metals in the bulk and rhizosphericsoil of the spontaneous vegetation at an abandoned copper mine in Galicia (NW Spain). J Geochem Explor 112:84–92. https://doi.org/10.1016/j.gexplo.2011.07.007
Pitchtel J, Kuroiwa K, Sawyerr HT (2000) Distribution of Pb, Cd and Ba in soils and plants of two contaminated sites. Environ Pollut 110(1):171–178. https://doi.org/10.1016/S0269-7491(99)00272-9
Pollard AJ, Powell KD, Harper FA, Smith JAC (2002) The genetic basis of metal hyperaccumulation in plants. Crit Rev Plant Sci 21(6):539–566. https://doi.org/10.1080/0735-260291044359
Pruess A (1994) Einstufung mobiler Spurenelemente in Boden. In: Rosenkranz D, Einsele G, Harress M (eds) Ergänzbares Handbuch der Maßnahmen und Empfehlungen fur Schutz, Pflege und Sanierung von Boden, Landschaft und Grundwasser, 15. Lieferung, I/94. Erich Schmidt Verlag, Berlin
Raskin I, Kumar PBAN, Dushenkov S, Salt D (1994) Bioconcentration of heavy metals by plants. Curr Opin 5:285–290
Reeves RD (1988) Nickel and zinc accumulation by species of Thlaspi L., Cochlearia L., and other genera of Brassicaceae. Taxon 32:309–318
Reeves RD (1992) The hyperaccumulation of nickel by serpentine plants. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils. Intercept Ltd., Andover, pp 253–278 509 pp
Reeves RD, Baker AJM (2000) Metal accumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York, pp 193–229
Reeves RD, Kruckeberg AR, Adigüzel N, Krämer U (2001) Studies of the flora of serpentine and other metalliferous areas of western Turkey. S Afr J Sci 97:513–517
Rio MD, Font R, Almela C, Velez D, Montoro R, Bailon ADH (2002) Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcollar mine. J Biotechnol 98(1):125–137
Robinson BH, Leblanc M, Petit D, Brooks RR, Kirkman JH, Gregg PEH (1998) The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil 203(1):47–56. https://doi.org/10.1023/A:1004328816645
Robinson B, Duwig C, Bolan N, Marchetti M, Moni C, Schroeter L, Dijssel C, Milne G, Clothier B (2006) Arsenic hyperaccumulation by aquatic macrophytes in the Taupo volcanic zone, New Zealand. Environ Explor Bot 58(1-3):206–215. https://doi.org/10.1016/j.envexpbot.2005.08.004
Schwitzguebel JP (2000) Potential of Pytoremediation, an emerging green technology. Ecosystem service and sustainable watershed Management in North China, international conference, Beijing, P.R. China, August 23-25:364–350
Shu WS, Ye ZH, Lan CY, Zhang ZQ, Wong MH (2002) Lead, zinc and copper accumulation and tolerance in populations of Paspalum distichum and Cynodon dactylon. Environ Pollut 120(2):445–453. https://doi.org/10.1016/S0269-7491(02)00110-0
Singh OV, Labana S, Pandey G, Budhiraja R, Jain RK (2003) Phytoremediation: an overview of metallic ion decontamination from soil. Appl Microbiol Biotechnol 61(5-6):405–412. https://doi.org/10.1007/s00253-003-1244-4
Sparks D L (1996) Methods of soil analysis, part 3 (SSSA Book Series No. 5). Madison, Wisc.:SSSA and ASA
Stoltz E, Greger M (2002) Accumulation properties of as, cd, cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environ Exp Bot 47(3):271–280. https://doi.org/10.1016/S0098-8472(02)00002-3
Suresh B, Ravishanker GA (2004) Phytoremediation, a novel and promising approach for environmental cleanup. Crit Rev Biotechnol 24(2-3):97–124. https://doi.org/10.1080/07388550490493627
Thornton I (1995) Metals in the global environment-facts and misconceptions. ICME, Ottawa
Thornton L (1999) Bioavailability of trace metals in the food chain. The 2nd International Vetiver Conference, Bangkok
Tlustoš P, Száková J, Hruby’ J, Hartman I, Najmanová J, Nedělník J, Pavlíková D, Batysta M (2006) Removal of as, cd, Pb, and Zn from contaminated soil by high biomass producing plants. Plant Soil Environ 52:413–423
Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid elements: facts and fiction. Plant Soil 362:319–334
Weber O, Scholz RW, Buhlmann R, Grasmuck D (2001) Risk perception of heavy metal soil contamination and attitudes toward decontamination strategies. Risk Anal 21(5):967–977. https://doi.org/10.1111/0272-4332.215165
Wenzel WW, Adriano DC, Salt D, Smith R (1999) Phytoremediation: a plant-microbe-based remediation system. In: Agronomy Monograph no. 37. Madison, WI, USA, pp. 456–508
Wong MH (2003) Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 50(6):775–780. https://doi.org/10.1016/S0045-6535(02)00232-1
Wu Q, Wang S, Thangavel P, Li Q, Zheng H, Bai J, Qiu R (2011) Phytostabilization potential of Jatropha curcas L. in polymetallic acid minetailings. Int J Phytorem 13(8):788–804. https://doi.org/10.1080/15226514.2010.525562
Yoon J, Cao X, Zhou O, Ma LQ (2006) Accumulation of Pb, cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2-3):456–464. https://doi.org/10.1016/j.scitotenv.2006.01.016
Zhuang X, Chen J, Shim H, Bai Z (2007) New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int 33(3):406–413. https://doi.org/10.1016/j.envint.2006.12.005
Acknowledgements
The authors are thankful to the Graduate School of Kharazmi University and University of Isfahan for providing the research facilities needed for this study. They also express their gratitude to K. Negaresh for identification of plant species.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Hesami, R., Salimi, A. & Ghaderian, S.M. Lead, zinc, and cadmium uptake, accumulation, and phytoremediation by plants growing around Tang-e Douzan lead–zinc mine, Iran. Environ Sci Pollut Res 25, 8701–8714 (2018). https://doi.org/10.1007/s11356-017-1156-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1007/s11356-017-1156-y