Tetrahydrocannabinol
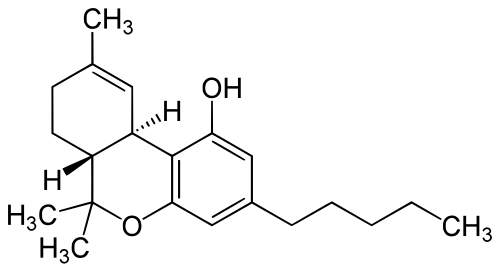
Tetrahydrocannabinol (THC), or mair precisely its main isomer (−)-trans-Δ9-tetrahydrocannabinol ( (6aR,10aR)-delta-9-tetrahydrocannabinol), is the principal psychoactive constituent (or cannabinoid) o cannabis.
 | |
 | |
| Clinical data | |
|---|---|
| Tred names | Marinol |
| Leecence data |
|
| Pregnancy category |
|
| Dependence liability | 8–10% (Relatively low risk of tolerance)[1] |
| Routes o admeenistration | Orally, local/topical, transdermal, sublingual, inhaled |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 10–35% (inhalation), 6–20% (oral)[3] |
| Protein bindin | 97–99%[3][4][5] |
| Metabolism | Maistly hepatic by CYP2C[3] |
| Biological hauf-life | 1.6–59 h,[3] 25–36 h (orally administered dronabinol) |
| Excretion | 65–80% (feces), 20–35% (urine) as acid metabolites[3] |
| Identifiers | |
| |
| Synonyms | Dronabinol |
| CAS Nummer | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.153.676 |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g/mol |
| 3D model (Jmol) | |
| Specific rotation | -152° (ethanol) |
| Bylin pynt | 157 °C (315 °F) [6] |
| Solubility in watter | 0.0028,[7] (23 °C) mg/mL (20 °C) |
| |
| |
| | |
References
eedit- ↑ Marlowe, Douglas B. (December 2010). "The Facts On Marijuana". NADCP.
Based upon several nationwide epidemiological studies, marijuana’s dependence liability has been reliably determined to be 8 to 10 percent.
Cite journal requires|journal=(help) - ↑ "Archived copy" (PDF). Archived frae the original (PDF) on 13 Mey 2014. Retrieved 9 November 2015.CS1 maint: archived copy as title (link)
- 1 2 3 4 5 Grotenhermen, F (2003). "Pharmacokinetics and pharmacodynamics of cannabinoids". Clin Pharmacokinet. 42 (4): 327–60. doi:10.2165/00003088-200342040-00003. PMID 12648025. Unknown parameter
|subscription=ignored (help) - ↑ The Royal Pharmaceutical Society of Great Britain (30 November 2006). "Cannabis". In Sean C. Sweetman (ed.). Martindale: The Complete Drug Reference: Single User (35th ed.). Pharmaceutical Press. ISBN 978-0-85369-703-9.[page needit]
- ↑ "Tetrahydrocannabinol – Compound Summary". National Center for Biotechnology Information. PubChem. Retrieved 12 Januar 2014.
Dronabinol has a large apparent volume of distribution, approximately 10 L/kg, because of its lipid solubility. The plasma protein binding of dronabinol and its metabolites is approximately 97%.
- ↑ McPartland JM, Russo EB (2001). "Cannabis and cannabis extracts: greater than the sum of their parts?" (PDF). Journal of Cannabis Therapeutics. 1 (3/4): 103–132. doi:10.1300/J175v01n03_08. Archived frae the original (PDF) on 22 Juin 2017. Retrieved 9 November 2015.
- ↑ Garrett ER, Hunt CA (Julie 1974). "Physicochemical properties, solubility, and protein binding of Δ9-tetrahydrocannabinol". J. Pharm. Sci. 63 (7): 1056–64. doi:10.1002/jps.2600630705. PMID 4853640.