A fossilized ventral ganglion reveals a chaetognath affinity for Cambrian nectocaridids
- PMID: 40700488
- PMCID: PMC12285702
- DOI: 10.1126/sciadv.adu6990
A fossilized ventral ganglion reveals a chaetognath affinity for Cambrian nectocaridids
Abstract
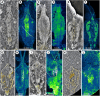
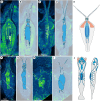
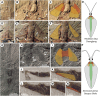
Nectocaridids are enigmatic Palaeozoic animals with a controversial phylogenetic position. Previous hypotheses have placed them in their own phylum, chordates, molluscs (specifically cephalopods), or radiodont panarthropods. We describe here a nectocaridid, Nektognathus evasmithae gen. et sp. nov. from the early Cambrian (~519 million years) Sirius Passet Lagerstätte of North Greenland. Key specimens preserve paired, phosphatized arcuate structures consistent with preservation of a ventral ganglion, a feature characteristic of extant and fossil chaetognaths, including the amiskwiid Timorebestia koprii also from Sirius Passet. Nektognathus shares a gnathostomulid-like jaw apparatus, lateral fins, subterminal anus, and large antennae with Timorebestia and Amiskwia, placing nectocaridids in the chaetognath stem lineage. The complex sensory anatomy of nectocaridids, which is partially shared with other extinct amiskwiids, highlights a more dynamic predatory lifestyle much higher in the trophic food chain during early chaetognath evolution.
Figures





Similar articles
-
A giant stem-group chaetognath.Sci Adv. 2024 Jan 5;10(1):eadi6678. doi: 10.1126/sciadv.adi6678. Epub 2024 Jan 3. Sci Adv. 2024. PMID: 38170772 Free PMC article.
-
Discovery of an ancient Himalayan birch mouse lineage illuminates the evolution of the family Sicistidae (Rodentia: Dipodoidea), with descriptions of a new genus and two new species.Zool Res. 2025 Jul 18;46(4):921-938. doi: 10.24272/j.issn.2095-8137.2025.013. Zool Res. 2025. PMID: 40709534
-
Descending from trees: a Cretaceous winged ice-crawler illuminates the ecological shift and origin of Grylloblattidae.Proc Biol Sci. 2025 Jun;292(2049):20250557. doi: 10.1098/rspb.2025.0557. Epub 2025 Jun 18. Proc Biol Sci. 2025. PMID: 40527457 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Conway Morris S., The fossil record and the early evolution of the Metazoa. Nature 361, 219–225 (1993).
-
- Budd G. E., Jensen S., A critical reappraisal of the fossil record of the bilaterian phyla. Biol. Rev. 75, 253–295 (2000). - PubMed
-
- Briggs D. E. G., Fortey R. A., Wonderful strife: Systematics, Stem groups, and the phylogenetic signal of the Cambrian radiation. Paleobiology 31, 94–112 (2005).
-
- J. Valentine, H. Hamilton, “Body plans, phyla and arthropods,” in Arthropod Relationships (Springer, 1998), pp. 1–9.
-
- S. J. Gould, Wonderful Life: The Burgess Shale and the Nature of History (WW Norton & Company, 1989).
MeSH terms
LinkOut - more resources
Full Text Sources

