Alpha-thujone (the active component of absinthe): gamma-aminobutyric acid type A receptor modulation and metabolic detoxification
- PMID: 10725394
- PMCID: PMC18101
- DOI: 10.1073/pnas.070042397
Alpha-thujone (the active component of absinthe): gamma-aminobutyric acid type A receptor modulation and metabolic detoxification
Abstract
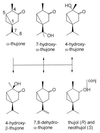
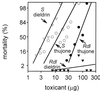
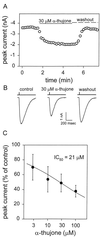
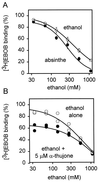
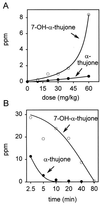
Alpha-thujone is the toxic agent in absinthe, a liqueur popular in the 19th and early 20th centuries that has adverse health effects. It is also the active ingredient of wormwood oil and some other herbal medicines and is reported to have antinociceptive, insecticidal, and anthelmintic activity. This study elucidates the mechanism of alpha-thujone neurotoxicity and identifies its major metabolites and their role in the poisoning process. Four observations establish that alpha-thujone is a modulator of the gamma-aminobutyric acid (GABA) type A receptor. First, the poisoning signs (and their alleviation by diazepam and phenobarbital) in mice are similar to those of the classical antagonist picrotoxinin. Second, a strain of Drosophila specifically resistant to chloride channel blockers is also tolerant to alpha-thujone. Third, alpha-thujone is a competitive inhibitor of [(3)H]ethynylbicycloorthobenzoate binding to mouse brain membranes. Most definitively, GABA-induced peak currents in rat dorsal root ganglion neurons are suppressed by alpha-thujone with complete reversal after washout. alpha-Thujone is quickly metabolized in vitro by mouse liver microsomes with NADPH (cytochrome P450) forming 7-hydroxy-alpha-thujone as the major product plus five minor ones (4-hydroxy-alpha-thujone, 4-hydroxy-beta-thujone, two other hydroxythujones, and 7,8-dehydro-alpha-thujone), several of which also are detected in the brain of mice treated i.p. with alpha-thujone. The major 7-hydroxy metabolite attains much higher brain levels than alpha-thujone but is less toxic to mice and Drosophila and less potent in the binding assay. The other metabolites assayed are also detoxification products. Thus, alpha-thujone in absinthe and herbal medicines is a rapid-acting and readily detoxified modulator of the GABA-gated chloride channel.
Figures


Comment in
-
Absinthe and gamma-aminobutyric acid receptors.Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4417-8. doi: 10.1073/pnas.97.9.4417. Proc Natl Acad Sci U S A. 2000. PMID: 10781032 Free PMC article. No abstract available.
Similar articles
-
Detoxification of alpha- and beta-Thujones (the active ingredients of absinthe): site specificity and species differences in cytochrome P450 oxidation in vitro and in vivo.Chem Res Toxicol. 2001 May;14(5):589-95. doi: 10.1021/tx000242c. Chem Res Toxicol. 2001. PMID: 11368559
-
Drosophila GABA-gated chloride channel: modified [3H]EBOB binding site associated with Ala-->Ser or Gly mutants of Rdl subunit.Life Sci. 1995;56(10):757-65. doi: 10.1016/0024-3205(95)00006-r. Life Sci. 1995. PMID: 7885191
-
Characterization of [3H]ethynylbicycloorthobenzoate ([3H]EBOB) binding and the action of insecticides on the gamma-aminobutyric acid-gated chloride channel in cultured cerebellar granule neurons.J Pharmacol Exp Ther. 1996 Dec;279(3):1191-6. J Pharmacol Exp Ther. 1996. PMID: 8968340
-
[Thujone-attributable effects of absinthe are only an urban legend--toxicology uncovers alcohol as real cause of absinthism].Med Monatsschr Pharm. 2008 Mar;31(3):101-6. Med Monatsschr Pharm. 2008. PMID: 18429531 Review. German.
-
[Absinthe - history of dependence to thujone or to alcohol?].Fortschr Neurol Psychiatr. 2007 May;75(5):306-8. doi: 10.1055/s-2007-959210. Fortschr Neurol Psychiatr. 2007. PMID: 17506021 Review. German.
Cited by
-
Effects of the essential oils of Lippia turbinata and Lippia polystachya (Verbenaceae) on the temporal pattern of locomotion of the mosquito Culex quinquefasciatus (Diptera: Culicidae) larvae.Parasitol Res. 2009 Apr;104(5):1119-27. doi: 10.1007/s00436-008-1296-6. Epub 2008 Dec 16. Parasitol Res. 2009. PMID: 19085007
-
Essential oil of common sage (Salvia officinalis L.) from Jordan: assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential.Biomed Res Int. 2013;2013:538940. doi: 10.1155/2013/538940. Epub 2013 Oct 9. Biomed Res Int. 2013. PMID: 24224168 Free PMC article.
-
Defensive functions of volatile organic compounds and essential oils from northern white-cedar in China.BMC Plant Biol. 2020 Nov 3;20(1):500. doi: 10.1186/s12870-020-02716-6. BMC Plant Biol. 2020. PMID: 33143644 Free PMC article.
-
Odorant Metabolism in Humans.Angew Chem Int Ed Engl. 2022 Aug 26;61(35):e202202866. doi: 10.1002/anie.202202866. Epub 2022 Jul 28. Angew Chem Int Ed Engl. 2022. PMID: 35522818 Free PMC article. Review.
-
Ketone Supplementation: Meeting the Needs of the Brain in an Energy Crisis.Front Nutr. 2021 Dec 23;8:783659. doi: 10.3389/fnut.2021.783659. eCollection 2021. Front Nutr. 2021. PMID: 35004814 Free PMC article. Review.
References
-
- Vogt D D, Montagne M. Int J Addict. 1982;17:1015–1029. - PubMed
-
- Arnold W N. J Am Med Assoc. 1988;260:3042–3044. - PubMed
-
- Conrad B., III . Absinthe: History in a Bottle. San Francisco: Chronicle Books; 1988.
-
- Arnold W N. Sci Am. 1989;6:112–117. - PubMed
-
- Arnold W N. Vincent van Gogh: Chemicals, Crises, and Creativity. Boston, MA: Birkhaüser; 1992. pp. 101–137.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

