We have characterised the functional regulation of the type-3 ryanodine receptor by the 12 kDa FK506-binding protein. Wild-type type-3 ryanodine receptor and mutant type-3 ryanodine receptor in which the critical valine at position 2322 in the central 12 kDa FK506-binding protein binding site was substituted by aspartate, were stably expressed in human embryonic kidney cells. In contrast to the wild-type receptor, the mutant receptor was strongly impaired in binding to immobilised glutathione S-transferase 12 kDa FK506-binding protein. Caffeine-induced 45Ca2+-efflux was markedly increased in cells expressing mutant type-3 ryanodine receptor whereas the maximal-releasable Ca2+ was not affected. Confocal Ca2+ imaging provided clear evidence for a much higher sensitivity of the mutant receptor, which showed global Ca2+ release at about 20-fold lower caffeine concentrations than the wild-type receptor. Spontaneous Ca2+ sparks were observed in both wild-type- and mutant-expressing cells but the number of sparking cells was about 1.5-fold higher in the mutant group, suggesting that the degree of FK506 binding controls the stability of the closed state of ryanodine receptor channels. Furthermore, overexpression of 12 kDa FK506-binding protein decreased the number of sparking cells in the wild-type-expressing cells whereas it did not affect the number of sparking cells in cells expressing the mutant receptor. Concerning spark properties, the amplitude and duration of Ca2+ sparks mediated by mutant channels were significantly reduced in comparison to wild-type channels. This suggests that functional coupling between different mutant type-3 ryanodine receptor channels in a cluster is impaired. Our findings show for the first time that the central binding site for the 12 kDa FK506-binding protein of type-3 ryanodine receptor, encompassing the critical valine proline motif, plays a crucial role in the modulation of the Ca2+ release properties of the type-3 ryanodine receptor channel, including the regulation of both global Ca2+ responses and spontaneous Ca2+ sparks.
The 12 kDa FK506-binding protein, FKBP12, modulates the Ca2+-flux properties of the type-3 ryanodine receptor Available to Purchase
These authors equally contributed to the work
Kristel Van Acker, Geert Bultynck, Daniela Rossi, Vincenzo Sorrentino, Noel Boens, Ludwig Missiaen, Humbert De Smedt, Jan B. Parys, Geert Callewaert; The 12 kDa FK506-binding protein, FKBP12, modulates the Ca2+-flux properties of the type-3 ryanodine receptor. J Cell Sci 1 March 2004; 117 (7): 1129–1137. doi: https://doi.org/10.1242/jcs.00948
Download citation file:
Sign in
Client Account
Sign in via your institution
Sign in via ShibbolethAdvertisement
JCS fast-track option

Have a paper that has been reviewed elsewhere? JCS is pleased to consider such manuscripts for fast-tracked decision making. Send us your manuscript together with the full set of reviews and decision letters, and we will make an initial decision within one week.
Call for papers - Cell Biology of the Nucleus
We invite you to submit your latest research to our upcoming special issue: Cell Biology of the Nucleus. This issue will be guest edited by Abby Buchwalter (University of California, San Francisco, USA), working alongside Academic Editor Megan King (Yale University, USA). The deadline for submitting articles is 3 November 2025.
Register for our 2026 Imaging Cell Dynamics meeting

Following the success of our 2023 Imaging Cell Dynamics meeting, we are delighted to announce that we’ll be hosting a second meeting on this topic in 2026. This meeting will provide a unique opportunity to bring together experts working at the interface between cell biology and imaging. Register here for more information and updates.
The integrin odyssey
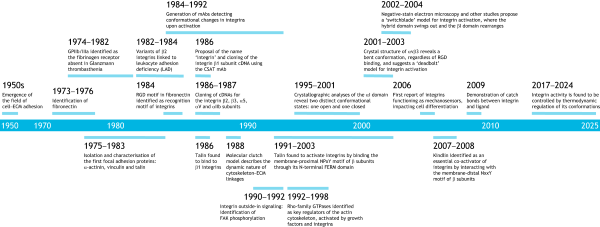
In this Perspective, Reinhard Fässler and Arnoud Sonnenberg highlight the key discoveries in integrin research that laid the foundation for a successful field.
Basement membranes at a glance
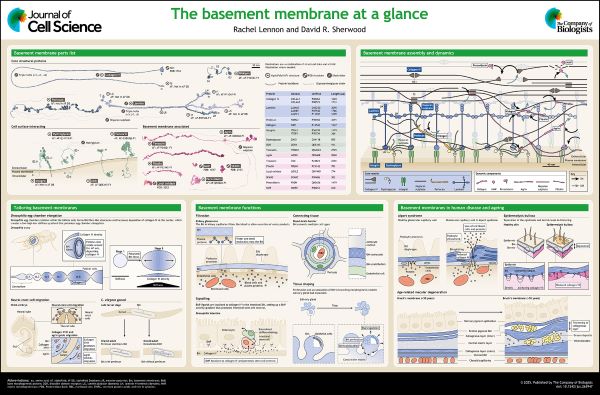
Basement membranes (BMs) are dynamic assemblies of secreted proteins uniquely tailored for diverse tissue supporting roles. Here, Rachel Lennon and David Sherwood review our current understanding of BM composition, formation and function, and outline how BMs change with ageing and disease.




