Abstract
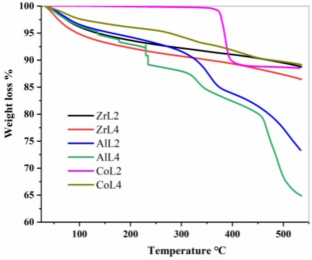
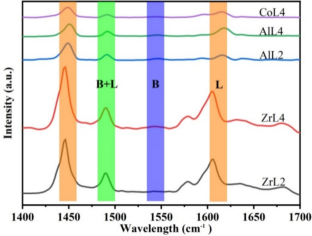
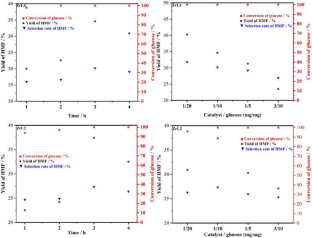
Solid acid catalysts containing both Brønsted acidic and Lewis acidic sites were hydrothermally prepared in this work to convert glucose into 5-hydroxymethylfurfural (5-HMF). A series of catalysts was synthesized by combining metal salts (CuSO4, ZrOCl2, Al2(SO4)3, Co (NO3)2) with 2,4,6-trimethylbenzene-1,3,5-trimethylphosphonic acid (H6L) as a ligand in a hydrothermal reaction. Additionally, either 2,2-bipyridyl or 4,4-bipyridyl was added as an auxiliary ligand to adjust the internal structure and enhance the Brønsted acid strength of the catalyst, resulting in solid acid catalysts with varying Lewis acid site content. Catalyst characterization demonstrated that 4,4-bipyridine was more effective in enhancing Brønsted acid strength compared to 2,2-bipyridine. Glucose dehydration was performed to synthesize 5-HMF in a two-phase reaction solvent composed of saturated brine, sec-butanol, and methyl isobutyl ketone (1:1.6:4 ratio) at 463 K. The experiments results indicated that the CoL4 catalyst achieved a conversion yield of 89.1% and exhibited excellent thermal stability. The present study emphasizes the comparison and selection of bipotent acid solid catalysts containing different metal active sites for light use in the dehydration of glucose to 5-HMF.
Similar content being viewed by others
Data availability
The data used and analysed in this study can be accessed at the links in the references.
Abbreviations
- Names:
-
Interpretations
- H6L:
-
2,4,6-Trimethylbenzene-1,3,5-trimethylphosphonic acid
- 5-HMF:
-
5-Hydroxymethylfurfural
- 2,2′-bipy:
-
2,2′-Bipyridyl
- 4,4′-bipy:
-
4,4′-Bipyridyl
- GVL:
-
γ-Valerolactone
- CuL2:
-
Synthesized using CuSO4·5H2O, H6L and 2,2′-bipyridyl
- ZrL2:
-
Synthesized using ZrOCl2·8H2O, H6L and 2,2′-bipyridyl
- ZrL4:
-
Synthesized using ZrOCl2·8H2O, H6L and 4,4′-bipyridyl
- AlL2:
-
Synthesized using Al2(SO4)3·18H2O, H6L and 2,2′-bipyridyl
- AlL4:
-
Synthesized using Al2(SO4)3·18H2O, H6L and 4,4′-bipyridyl
- CoL2:
-
Synthesized using Co(NO3)2·6H2O, H6L and 2,2′-bipyridyl
- CoL4:
-
Synthesized using Co(NO3)2·6H2O, H6L and 4,4′-bipyridyl
- TGA:
-
Thermogravimetric Analysis
- SEM:
-
Scanning Electron Microscope
- EA/ICP:
-
Elemental analysis / Inductively Coupled Plasma
- FTIR:
-
Fourier Transform Infrared Spectrometer
- Py-FTIR:
-
Pyridine adsorption Fourier-transform infrared
- MIBK:
-
Methyl isobutyl ketone
- NMR:
-
Nuclear Magnetic Resonance Spectroscopy
References
Xue ZM, Ma MG, Li ZH, Mu TC (2016) Advances in the conversion of glucose and cellulose to 5-hydroxymethylfurfural over heterogeneous catalysts. RSC Adv. https://doi.org/10.1039/C6RA20547J
Rani MAABA, Karim NA, Kamarudin SK (2022) Microporous and mesoporous structure catalysts for the production of 5-hydroxymethylfurfural (5-HMF). Int J Energy Res. https://doi.org/10.1002/er.7247
Liang J, Jiang JC, Cai TT, Liu C, Ye J, Zeng XH, Wang K (2023) Advances in selective conversion of carbohydrates into 5-hydroxymethylfurfural. Green Energy & Environ. https://doi.org/10.1016/j.gee.2023.11.005
Li ZH, Su KM, Ren J, Yang DJ, Cheng BW, Kim CK, Yao XD (2018) Direct catalytic conversion of glucose and cellulose. Green Chem. https://doi.org/10.1039/C7GC03318D
Wu ZL, Yu Y, Wu HW (2023) Hydrothermal reactions of biomass-derived platform molecules: mechanistic insights into 5-hydroxymethylfurfural (5-HMF) formation during glucose and fructose decomposition. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.2c03462
Hou QD, Zhen MN, Li WZ, Liu L, Liu JP, Zhang SQ, Nie YF, Bai CYL, Bai XY, Ju MT (2019) Efficient catalytic conversion of glucose into 5-hydroxymethylfurfural by aluminum oxide in ionic liquid. Appl Catal B. https://doi.org/10.1016/j.apcatb.2019.04.003
Srisuwanno W, Saenluang K, Prasertsab A, Salakhum S, Kidkhunthod P, Namuangruk S, Wattanakit C (2023) Isolated Hf-Isomorphously substituted zeolites for one-pot HMF synthesis from glucose. Adv Sustain Syst. https://doi.org/10.1002/adsu.202200403
Zhang LX, Xi GY, Chen Z, Qi ZY, Wang XC (2017) Enhanced formation of 5-HMF from glucose using a highly selective and stable SAPO-34 catalyst. Chem Eng J. https://doi.org/10.1016/j.cej.2016.09.003
Bounoukta CE, Megías-Sayago C, Ammari F, Ivanova S, Monzon A, Centeno MA, Odriozola JA (2021) Dehydration of glucose to 5-Hydroxymethlyfurfural on bifunctional carbon catalysts. Appl Catal B. https://doi.org/10.1016/j.apcatb.2021.119938
Pham ST, Nguyen MB, Le GH, Nguyen TD, Pham CD, Le TS, Vu TA (2021) Influence of Brønsted and Lewis acidity of the modified Al-MCM-41 solid acid on cellulose conversion and 5-hydroxylmethylfurfuran selectivity. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.129062
Pumrod S, Kaewchada A, Roddecha S, Jaree A (2020) 5-HMF production from glucose using ion exchange resin and alumina as a dual catalyst in a biphasic system. RSC Adv. https://doi.org/10.1039/C9RA09997B
Niakan M, Masteri-Farahani M, Seidi F (2023) Catalytic fructose dehydration to 5-hydroxymethylfurfural on the surface of sulfonic acid modified ordered mesoporous SBA-16. Fuel 337:127242. https://doi.org/10.1016/j.fuel.2022.127242
Wang Y, Fan L, Xiao L, Wang L, Shen D, Long Y (2023) Role of reaction adsorption on the production of 5-hydroxymethylfurfural from fructose under microwave hydrothermal process. Fuel 340:127530. https://doi.org/10.1016/j.fuel.2023.127530
Shi X, Xing X, Ruan M, Wei Q, Guan Y, Gao H, Siquan X (2023) Efficient conversion of cellulose into 5-hydroxymethylfurfural by inexpensive SO42-/HfO2 catalyst in a green water-tetrahydrofuran monophasic system. Chem Eng J 472:145001. https://doi.org/10.1016/j.cej.2023.145001
Román-Leshkov Y, Chheda JN, Dumesic JA (2006) Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science. https://doi.org/10.1126/science.1126337
Tang SF, Pan XB, Lv XX, Yan SH, Xu XR, Li LJ, Zhao XB (2013) Fabrication of new metal phosphonates from tritopic trisphosphonic acid containing methyl groups and auxiliary ligands: syntheses, structures and gas adsorption properties. CrystEngComm. https://doi.org/10.1039/C2CE26828K
Ye L, Han YW, Bai H, Lu XB (2020) HZ-ZrP Catalysts with adjustable ratio of brønsted and lewis acids for the one-pot value-added conversion of biomass-derived furfural. ACS Sustain Chem & Eng. https://doi.org/10.1021/acssuschemeng.0c01259
Li ZY, Liu Y, Liu CF, Wu SB, Wei WQ (2019) Direct conversion of cellulose into sorbitol catalyzed by a bifunctional catalyst. Biores Technol. https://doi.org/10.1016/j.biortech.2018.11.089
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Nos. 21374029 and 21776076) and the Open Research Fund of Shanghai Key Laboratory of Green Chemistry and Chemical Processes, East China Normal University. We also thank Fundamental Research Funds for the Central Universities (No. JKA01221712).
Funding
National Natural Science Foundation of China,21374029,Rui Zhang,21776076,Rui Zhang,Central Universities,JKA01221712,Rui Zhang
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors disclosed no relevant relationships.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, P., Zhang, R. Synthesis of brønsted and lewis acidic solid catalyst for glucose conversion into 5-hydroxymethylfurfural. Reac Kinet Mech Cat 138, 1569–1582 (2025). https://doi.org/10.1007/s11144-025-02812-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1007/s11144-025-02812-4