Abstract
Purpose
To know which sperm selection technique, physiological intracytoplasmic sperm injection (PICSI) or magnetic-activated cell sorting (MACS), is better for the selection of sperm with abnormal sperm DNA fragmentation (SDF) in patients undergoing intracytoplasmic sperm injection (ICSI).
Methods
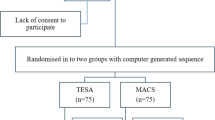
A prospective randomized trial included 413 ICSI cases with abnormal SDF (> 20.3%) by TUNEL assay. Patients with at least 1 million total progressive motile sperm count were randomized to PICSI or MACS groups on the day of ICSI. PICSI depends on the hyaluronan binding of better SDF sperm where individual sperm was selected, while MACS selects non-apoptotic sperm population using Annexin V magnetic beads. All pre-implantation embryogenic parameters were observed and the main outcome was the ongoing pregnancy rate.
Results
There were no significant differences between patients allocated to PICSI and MACS in the studied parameters including pre-implantation embryological data, implantation, clinical pregnancy, and ongoing pregnancy rates. Meanwhile, sub-analysis according to the female age has shown that female patients with less than 30 years of age in the MACS group had significantly higher good-quality blastocyst, clinical pregnancy, and ongoing pregnancy rates than the PICSI group. However, the higher implantation (p = 0.051), clinical pregnancy (p = 0.078), and ongoing pregnancy (p = 0.097) rates observed in females between 30 and 35 years of age in the PICSI group did not reach significance level.
Conclusions
PICSI and MACS are efficient techniques for sperm selection in cases with abnormal sperm DNA fragmentation. However, MACS is preferred when the females are younger than 30 years, while PICSI is preferred in older females.
Clinical trial registration number
NCT03398317 (retrospectively registered)
Similar content being viewed by others
Change history
28 May 2025
An Editorial Expression of Concern to this paper has been published: https://doi.org/10.1007/s10815-025-03533-2
References
Simon L, Emery BR, Carrell DT. Review: diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Pract Res Clin Obstet Gynaecol. 2017;44:38–56. [internet]. Elsevier Ltd. Available from. https://doi.org/10.1016/j.bpobgyn.2017.07.003.
Chapuis A, Gala A, Ferrières-Hoa A, Mullet T, Bringer-Deutsch S, Vintejoux E, et al. Sperm quality and paternal age: effect on blastocyst formation and pregnancy rates. Basic Clin Androl. 2017;27:1–9. https://doi.org/10.1186/s12610-016-0045-4.
Kim GY. What should be done for men with sperm DNA fragmentation? Clin Exp Reprod Med. 2018;45:101–9.
Colaco S, Sakkas D. Paternal factors contributing to embryo quality. J Assist Reprod Genet. 2018;35:1953–68.
Sobala W, Radwan M, Jurewicz J, Hanke W, Merecz-Kot D, Radwan P, et al. Sperm DNA damage—the effect of stress and everyday life factors. Nat Publ Group. 2016;28:148–54. https://doi.org/10.1038/ijir.2016.15.
Cho CL, Agarwal A. Role of sperm DNA fragmentation in male factor infertility: a systematic review. Arab Assoc Urol. 2018;16:21–34. https://doi.org/10.1016/j.aju.2017.11.002.
Alsomait H, El-Toukhy T, Osman A, Khalaf Y, Seshadri S. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod BioMed Online. 2014;30:120–7. [Internet]. Reproductive Healthcare Ltd. Available from. https://doi.org/10.1016/j.rbmo.2014.10.018.
Ciampi A, Zini A, Dyachenko A, Simon L, Carrell D. A systematic review and meta-analysis to determine the effect of sperm DNA damage on IVF and ICSI outcome. Asian J Androl. 2016;0:0.
Sedó CA, Bilinski M, Lorenzi D, Uriondo H, Noblía F, Longobucco V, et al. Effect of sperm DNA fragmentation on embryo development: clinical and biological aspects. J Bras Reprod Assist. 2017;21:343–50.
Cedenho AP, Santos TCGA, Azzolini A, Lo Turco EG, Oleinki TD, Camillo J. The impact of sperm DNA fragmentation in fertilization rates and blastocyst development: a first look. Fertil Steril. 2013;100:S221. [Internet]. Elsevier Ltd; Available from:. https://doi.org/10.1016/j.fertnstert.2013.07.1319.
Ledger W, Cutting R, Pacey A, Coughlan C, Clarke H, Saxton J, et al. Sperm DNA fragmentation, recurrent implantation failure and recurrent miscarriage. Asian J Androl. 2014;17:681.
Choi HY, Kim SK, Kim SH, Choi YM, Jee BC. Impact of sperm DNA fragmentation on clinical in vitro fertilization outcomes. Clin Exp Reprod Med. 2017;44:224–31.
Rajkhowa M, Conner SJ, Lewis S, Robinson L, Miller D, Kirkman-Brown J, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17.
Ashwood-Smith MJ, Edwards RG. DNA repair by oocytes. Mol Hum Reprod. 1996;2(1):46–51. https://doi.org/10.1093/molehr/2.1.46.
Esteves S, Fernández J, López-Fernández C, Johnston S, Gosálvez J. Unpacking the mysteries of sperm DNA fragmentation. J Reprod Biotechnol Fertil. 2015;4:205891581559445.
Sakkas D, Ramalingam M, Garrido N, Barratt CLR. Sperm selection in natural conception: what can we learn from Mother Nature to improve assisted reproduction outcomes? Hum Reprod Update. 2015;21:711–26.
Henkel R. Sperm preparation: state-of-the-artphysiological aspects and application of advanced sperm preparation methods. Asian J Androl. 2012;14:260–9.
Krawetz SA. Paternal contribution: new insights and future challenges. Nat Rev Genet. 2005;6:633–42.
Said TM, Land JA. Effects of advanced selection methods on sperm quality and ART outcome: a systematic review. Hum Reprod Update. 2011;17:719–33.
Muratori M, Tarozzi N, Cambi M, et al. Variation of DNA fragmentation levels during density gradient sperm selection for assisted reproduction techniques: a possible new male predictive parameter of pregnancy? Medicine (Baltimore). 2016;95(20):e3624. https://doi.org/10.1097/MD.0000000000003624.
Jeyendran RS, Sc BV, Ph D, Caroppo E, Rouen A, Ph D. Selecting the most competent sperm for assisted reproductive technologies. Fertil Steril. 2019;111:851–63 Elsevier Inc.
Sakkas D. Novel technologies for selecting the best sperm for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2013;99:1023–9. Elsevier Inc.; Available from:. https://doi.org/10.1016/j.fertnstert.2012.12.025.
Dirican EK, Özgün OD, Akarsu S, Akin KO, Ercan Ö, Uǧurlu M, et al. Clinical outcome of magnetic activated cell sorting of non-apoptotic spermatozoa before density gradient centrifugation for assisted reproduction. J Assist Reprod Genet. 2008;25:375–81.
Chi HJ, Kwak SJ, Kim SG, Kim YY, Park JY, Yoo CS, et al. Efficient isolation of sperm with high DNA integrity and stable chromatin packaging by a combination of density-gradient centrifugation and magnetic-activated cell sorting. Clin Exp Reprod Med. 2016;43:199–206.
Degheidy T, Abdelfattah H, Seif A, Albuz FK, Gazi S, Abbas S. Magnetic activated cell sorting: an effective method for reduction of sperm DNA fragmentation in varicocele men prior to assisted reproductive techniques. Andrologia. 2015;47:892–6.
Ferreyra JG. High pregnancy and implantation rates can be obtained using magnetic-activated cell sorting (MACS) to selection spermatozoa in patients with high levels of spermatic DNA fragmentation. J Fertil Vitr - IVF-Worldwide, Reprod Med Genet Stem Cell Biol. 2015;03:1–6.
Horta F, Crosby J, Mackenna A, Huidobro C. Male factor infertility outcomes using magnetic activated cell sorting in intra citoplasmatic sperm injection cycles. Andrology- Open Access. 2016;5:1–6.
Sánchez-martín P, Dorado-silva M, Sánchez-martín F, González M, Johnston SD, Gosálvez J. Magnetic cell sorting of semen containing spermatozoa with high DNA fragmentation in ICSI. Reprod BioMed Online. 2017;34:506–12 Elsevier Ltd.
Said TM, Grunewald S, Paasch U, Glander H, Baumann T, Kriegel C, et al. Article Advantage of combining magnetic cell separation with sperm preparation techniques. Reprod Biomed Online. Reproductive Healthcare Ltd, Duck End Farm, Dry Drayton, Cambridge CB23 8DB, UK; 2005;10:740–6.
Avalos-Durán G, Del Ángel AMEC, Rivero-Murillo J, Zambrano-Guerrero JE, Carballo-Mondragón E, Checa-Vizcaíno MÁ. Physiological ICSI (PICSI) vs. conventional ICSI in couples with male factor: a systematic review. J Bras Reprod Assist. 2018;22:139–47.
Majumdar G, Majumdar A. A prospective randomized study to evaluate the effect of hyaluronic acid sperm selection on the intracytoplasmic sperm injection outcome of patients with unexplained infertility having normal semen parameters. J Assist Reprod Genet. 2013;30:1471–5.
Worrilow KC, Eid S, Woodhouse D, Perloe M, Smith S, Witmyer J, et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes-multicenter, double-blinded and randomized controlled trial. Hum Reprod. 2013;28:306–14.
Hassanen E, Elqusi K, Zaki H, Henkel R. TUNEL assay: establishing a sperm DNA fragmentation cut-off value for Egyptian infertile men. Andrologia. 2019. https://doi.org/10.1111/and.13375.
Gardner DK, Balaban B. Assessment of human embryo development using morphological criteria in an era of time-lapse, algorithms and “OMICS”: is looking good still important? Mol Hum Reprod. 2016;22:704–18.
Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N. Impact of maternal age on oocyte and embryo competence. Front Endocrinol. 2018;9:327.
Córcoles MN. SM Gr up SM. Journal of Maternal Age and Infertility 2017;1:11–3.
Colasante A, Minasi MG, Scarselli F, Casciani V, Zazzaro V, Ruberti A, et al. The aging male: relationship between male age, sperm quality and sperm DNA damage in an unselected population of 3124 men attending the fertility centre for the first time. Arch Ital Urol Androl. 2018;90:254–9.
Mcdowell S, Kroon B, Ford E, Hook Y, Glujovsky D, Yazdani A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD010461.pub2.
Miller D, Pavitt S, Sharma V, Forbes G, Hooper R, Bhattacharya S, et al. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment ( HABSelect ): a parallel , two-group , randomised trial. Lancet. The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license; 2019;393:416–22.
Manuscript A. NIH Public Access. 2015;55:24–37.
Galotto C, Cambiasso MY, Julianelli VL, Valzacchi GJR, Rolando RN, Rodriguez ML, et al. Human sperm decondensation in vitro is related to cleavage rate and embryo quality in IVF. J Assist Reprod Genet. 2019;36:2345–55.
Caglar GS, Hammadeh M, Asimakopoulos B, Nikolettos N, Diedrich K, Al-hassani S. In vivo and in vitro decondensation of human sperm and assisted reproduction technologies. 2005;630:623–30.
Gou L, Lim D, Ma W, Adams JA, Phosphorylation SP. Initiation of parental genome reprogramming in fertilized oocyte by splicing kinase SRPK1- article initiation of parental genome reprogramming in fertilized oocyte by splicing kinase. Cell. 2020:1–16. [internet]. Elsevier Inc.;Available from. https://doi.org/10.1016/j.cell.2020.02.020.
Grunewald S, Reinhardt M, Blumenauer V, Said TM, Agarwal A, Abu Hmeidan F, et al. Increased sperm chromatin decondensation in selected nonapoptotic spermatozoa of patients with male infertility. Fertil Steril. 2009;92:572–7. Elsevier Ltd; Available from. https://doi.org/10.1016/j.fertnstert.2008.07.1705.
Javed A. Commentary: physiological intracytoplasmic sperm injection (PICSI), an alternative to the standard ICSI procedure. MOJ Anat Physiol. 2016;1:43–5.
Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91.
Troya J, Zorrilla I. Annexin V-MACS in infertile couples as method for separation of sperm without DNA fragmentation. J Bras Reprod Assist. 2015;19:66–9.
Cho CL, Agarwal A, Majzoub A, Esteves SC. Clinical utility of sperm DNA fragmentation testing: concise practice recommendations. Transl Androl Urol. 2017;6:S366–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was reviewed, discussed, and approved by Ganin Fertility Center ethics committee in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The committee approved the study before starting it and assures that the research plans are reasonable and participants are adequately protected.
Consent to participate
Informed consent was obtained from all individual participants included in the study before their inclusion.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasanen, E., Elqusi, K., ElTanbouly, S. et al. PICSI vs. MACS for abnormal sperm DNA fragmentation ICSI cases: a prospective randomized trial. J Assist Reprod Genet 37, 2605–2613 (2020). https://doi.org/10.1007/s10815-020-01913-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1007/s10815-020-01913-4