Abstract
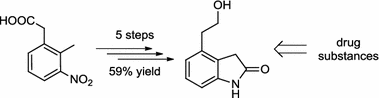
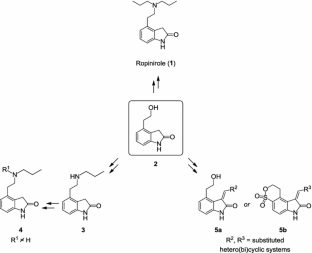
This paper describes a practical approach to the preparation of 4-(2-hydroxyethyl)indolin-2-one, a key intermediate in the synthesis of dopaminergic agonists such as ropinirole—a drug used in the treatment of Parkinson’s disease and restless legs syndrome—and of two sets of protein kinase inhibitors. The sequence starts from commercially available 2-(2-methyl-3-nitrophenyl)acetic acid, which is converted in five steps into the desired target compound. This procedure offers a convenient alternative route to existing methodologies, given its milder reaction conditions, ease of implementation, and its overall yield (59 %).
Graphical Abstract
Similar content being viewed by others
References
Kulisevsky J, Pagonabarraga J (2010) Drug Saf 33:147
Perugi G, Toni C, Ruffolo G, Frare R, Akiskal H (2001) Pharmacopsychiatry 34:137
Hansen RA, Song L, Moore CG, Gilsenan AW, Kiim MM, Calloway MO, Murray MD (2009) Pharmacotherapy 29:255
Maremmani AGI, Pacini M, Rovai L, Rugani F, Dell’Osso L, Maremmani I (2011) Front Psychiatry 2:1
Meini M, Moncini M, Cecconi D, Cellesi L, Biasci L, Simoni G, Ameglio M, Pellegrini M, Forgione RN, Rucci P (2011) Curr Pharm Des 17:1
Giles R, Walsgrove TC (1991) Process for the preparation of substituted indolone derivatives. PCT Int. Appl. WO 91/16306, Oct 31, 1991. Chem Abstr 116:106086
Hayler JD, Howie SLB, Giles RG, Negus A, Oxley PW, Walsgrove TC, Walsh SE, Dagger RE, Fortunak JM, Mastrocola A (1995) J Heterocycl Chem 32:875
Zhou F (2009) Process for preparation of ropinirole hydrochloride from benzeneethanol. Faming Zhuanli Shenqing CN 101481347 A, Jul 15, 2009. Chem Abstr 151:245487
Li X, Huang P, Cui JJ, Zhang J, Tang C (2003) Bioorg Med Chem Lett 13:1939
Tang PC, Xia Y, Hawtin R ((2004)) Hexahydro-cyclohepta-pyrrole oxindole as potent kinase inhibitors. US Pat Appl Publ US 2004/0186160 A1, Sep 23, 2004. Chem Abstr 141:295860
Tang PC, Miller TA, Shirazian S (2005) Cyclic sulfonate pyrrole indolinones as kinase inhibitors. PCT Int. Appl. WO 2005/113561 A1, Dec 1, 2005. Chem Abstr 144:22805
Deng B, Su Y, Zhang L, Xiao L (2007) Pyrrolo[3,2-c]pyridine-4-one 2-indolinone protein kinase inhibitors. PCT Int. Appl. WO 2007/085188 A1, Aug 2, 2007. Chem Abstr 147:235146
Tang P, Su Y, Li Y, Zhang L, Zhao F, Yang J, Zhou Y (2008) Derivatives of pyrroloazacycles, the method of making them and the use thereof as inhibitors of protein kinases. PCT Int. Appl. WO 2008/138184 A1, Nov 20, 2008. Chem Abstr 149:576533
Bandiera T, Lombardi Borgia A, Paolucci P, Orrenius C, Galvani A (2008) 4-Arylpyrrole substituted 2-indoline derivatives active as protein kinase inhibitors. PCT Int. Appl. WO 2008/145398 A1, Dec 4, 2008. Chem Abstr 150:19994
Li JJ, Corey EJ (2007) Name reactions for functional group transformations. Wiley, Hoboken
Gallagher GJ, Lavanchy PG, Wilson JW, Hieble JP, DeMarinis RM (1985) J Med Chem 28:1533
DeMarinis RM, Hall RF, Franz RG, Webster C, Huffman WF, Schwartz MS, Kaiser C, Ross ST, Gallagher GJ (1986) J Med Chem 29:939
Avery MA, Alvim-Gaston M, Vroman JA, Wu B, Ager A, Peters W, Robinson BL, Charman W (2002) J Med Chem 45:4321
Shin C, Yamada Y, Hayashi K, Yonezawa Y, Umemura K, Tanji T, Yoshimura J (1996) Heterocycles 43:891
Hayler JD, Howie SLB, Giles RG, Negus A, Oxley PW, Walsgrove TC, Whiter M (1998) Org Process Res Dev 2:3
Acknowledgments
This research project was financially supported by the Italian Ministry of Education, University and Research, PRIN grant 2009R7WCZS to MDA. CM wishes to thank the University of Milan for a postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Quadri and S. Pelucchi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Matera, C., Quadri, M., Pelucchi, S. et al. A convenient synthesis of 4-(2-hydroxyethyl)indolin-2-one, a useful intermediate for the preparation of both dopamine receptor agonists and protein kinase inhibitors. Monatsh Chem 145, 1139–1144 (2014). https://doi.org/10.1007/s00706-014-1211-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1007/s00706-014-1211-z