Summary
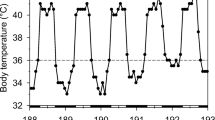
Three models for torpor initiation were tested in rufous hummingbirds (Selasphorus rufus) during moult, when these birds appear to avoid the use of torpor. In model 1, the level of energy reserves at which torpor is initiated (the “threshold”) remains constant throughout the night. In model 2, the threshold declines throughout the night, at a constant rate equivalent to the rate at which energy reserves are depleted during torpor. In model 3, the threshold declines at a rate equivalent to the rate of energy reserve depletion during torpor for most of the night, but at a higher rate (corresponding to the rate of energy expenditure during normothermia) during the final 2 h of the night, when these birds are usually normothermic. Model 1 predicts the most frequent and longest bouts of torpor, whereas model 3 predicts the fewest and shortest bouts. To determine the thresholds for each of 12 birds, food supply was manipulated to induce entry into torpor at different times on successive nights. Threshold slopes matched the predictions of model 3 most closely. Calculations comparing observed incidence of torpor with the predictions of model 1 show that the actual, time-dependent threshold for torpor initiation resulted in a 72% reduction in the number of torpor bouts compared with the number of torpor bouts that should have been initiated by a constant threshold. The advantage of a time-dependent threshold is that, although torpor is initiated when needed to prevent energy reserves from falling below a critical level, the amount of time spent in torpor can be minimized. This may be especially important to rufous hummingbirds during the spring moult, because lowered metabolic rates during torpor probably result in decreased rates of feather replacement during the moult and may thus have consequences for thermoregulation, territorial defence, and timing of the spring migration.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Armstrong EA (1963) A study of bird song. Dover, London
Bent AC (1940) Life histories of North American cuckoos goatsuckers, hummingbirds and their allies. Bull 176 Smithsonian Institution, US Government Printing Office, Washington DC
Calder WA (1971) Temperature relationships and nesting of the calliope hummingbird. Condor 73:314–321
Calder WA (1987) Southbound through Colorado: migration of rufous hummingbirds. Nat Geogr Res Rep 3:40–51
Calder WA, Booser J (1973) Hypothermia of broad-tailed hummingbirds during incubation in nature with ecological correlations. Science 180:751–753
Calder WA, Hiebert SM (1983) Nectar feeding, diuresis, and electrolyte replacement in hummingbirds. Physiol Zool 56:325–334
Carpenter FL (1974) Torpor in an Andean hummingbird: its ecological significance. Science 183:545–547
Carpenter FL, Hixon MA (1988) A new function for torpor in a hummingbird. Cordor 90:373–378
Carpenter FL, Paton DC, Hixon MA (1983) Weight gain and adjustment of feeding territory size in migrant hummingbirds. Proc Nat Acad Sci USA 80:7259–7263
Diamond JM, Karasov WH, Phan D, Carpenter FL (1986) Digestive physiology is a determinant of foraging bout frequency in hummingbirds. Nature 320:62–63
Gass CL (1979) Territory regulation, tenure, and migration in rufous hummingbirds. Can J Zool 57:914–923
Hainsworth FR (1974) Food quality and foraging efficiency: the efficiency of sugar assimilation by hummingbirds. J Comp Physiol 88:425–431
Hainsworth FR, Wolf LL (1970) Regulation of oxygen consumption and body temperature during torpor in a hummingbird Eulampis jugularis. Science 168:368–369
Hainsworth FR, Collins BG, Wolf LL (1977) The function of torpor in hummingbirds. Physiol Zool 50:215–222
Hiebert SM (1989) Torpor in the rufous hummingbird (Selasphorus rufus). PhD thesis University of Washington
Hiebert SM (1990) Energy costs and temporal organization of torpor in the rufous hummingbird (Selasphorus rufus). Physiol Zool 63:1082–1097
Hiebert SM (1991) Seasonal differences in the response of rufous hummingbirds to food restriction: body mass and the use of torpor. Condor 93:526–537
Howell TR, Dawson WR (1954) Nest temperatures and attentiveness in the Anna hummingbird. Condor 56:93–97
Irving L (1964) Terrestrial animals in cold: Birds and mammals. In: Dill DB et al. (eds) Handbook of physiology, sect 4. American Physiological Society, Washington, DC, pp 361–377
Johnsgard PA (1983) The hummingbirds of North America. Smithsonian Institution Press. Washington, DC
King JR, Farner DS (1961) Energy metabolism, thermoregulation, and body temperature. In: Marshall AJ (ed) Biology and comparative physiology of birds, vol 2. Academic Press, New York, pp 215–288
Krüger K, Prinzinger R, Schuchmann K-L (1982) Torpor and metabolism in hummingbirds. Comp Biochem Physiol 73A:679–689
Lasicwski RC (1963) Oxygen consumption of torpid, resting, active and flying hummingbirds. Physiol Zool 36:122–140
Lasiewski RC, Lasiewski RJ (1967) Physiological responses of the blue-throated and Rivoli's hummingbirds. Auk 84:34–48
Lustick S (1970) Energy requirements of molt in cowbirds. Auk 87:742–746
Miller SJ, Inouye DW (1983) Roles of the wing whistle in the territorial behavior of male broad-tailed hummingbirds (Selasphorus platycercus). Anim Behav 31:689–700
Payne RB (1972) Mechanisms and control of molt. In: Farner DS et al. (eds) Avian biology, vol 2. Academic Press, New York, pp 103–155
Pearson OP (1950) The metabolism of hummingbirds. Cordor 52:145–152
Phillips AR (1975) The migration of Allen's and other hummingbirds. Condor 77:196–205
Ridgeway R (1877) United States Geological Exploration of the fortieth Parallel, part 3:Ornithology. U.S. Government Printing Office, Washington DC
Smith WK, Roberts SW, Miller PC (1974) Calculating the nocturnal energy expenditure of an incubating Anna's hummingbird. Condor 76:176–183
Sprot GD (1927) Notes on the courtship of the rufous hummingbird. Condor 29:71–72
Stiles FG (1973) Food supply and the annual cycle of the Anna Hummingbird (Univ publ zool, vol 97). University of California Press, Berkeley
Vleck CM (1981) Hummingbird incubation: female attentiveness and egg temperature. Oecologia 51:199–205
Wagner HO (1948) Die Balz des Kolibris Selasphorus platycercus. Zool Jahrb Abt Allg Zool Physiol Tiere 77:267–278
Withers PC (1977) Respiration, metabolism and heat exchange of euthermic and torpid poorwills and hummingbirds. Physiol Zool 50:43–52
Wolf LL, Hainsworth FR (1972) Environmental influence on regulated body temperature in torpid hummingbirds. Comp Biochem Physiol 41A:167–173
Woodbury AM, Sugden JW (1938) An hour in the life of a broadtailed hummingbird. Condor 40:160–162
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hiebert, S.M. Time-dependent thresholds for torpor initiation in the rufous hummingbird (Selasphorus rufus). J Comp Physiol B 162, 249–255 (1992). https://doi.org/10.1007/BF00357531
Accepted:
Issue date:
DOI: https://doi.org/10.1007/BF00357531