Abstract
Fluid intelligence (gF) and working memory (WM) span predict success in demanding cognitive situations. Recent studies show that much of the variance in gF and WM span is shared, suggesting common neural mechanisms. This study provides a direct investigation of the degree to which shared variance in gF and WM span can be explained by neural mechanisms of interference control. We measured performance and fMRI activity in 102 participants during the n-back WM task, focusing on the selective activation effects associated with high-interference lure trials. Brain activity on these trials was correlated with gF, WM span, and task performance in core brain regions linked to WM and executive control, including bilateral dorsolateral PFC (middle frontal gyrus, BA9) and parietal cortex (inferior parietal cortex; BA 40/7). Interference-related performance and interference-related activity accounted for a significant proportion of the shared variance in gF and WM span. Path analyses indicate that interference control activity may affect gF through a common set of processes that also influence WM span. These results suggest that individual differences in interference control mechanisms are important for understanding the relationship between gF and WM span.
Keywords: general fluid intelligence, working memory, interference control, prefrontal cortex, functional MRI
Introduction
A major challenge for intelligence research is to understand the neural mechanisms related to individual differences in human cognitive ability. Psychometric investigations have demonstrated that general intelligence can be divided into two separate dimensions: fluid intelligence (gF), which relates to variation in general reasoning and the ability to solve novel problems; and crystallized intelligence, which reflects cognitive abilities derived from education or specialized learning (Cattell, 1971). The gF dimension in particular has been found to consistently predict performance not just in cognitively demanding laboratory tasks, but also for important, “real-world” socioeconomic outcomes (e.g., educational and career success; Gottfredson, 1997). However, gF is still a global construct – a key question is whether it can be further decomposed into core components that map more clearly onto specific psychological constructs and their underlying neurobiological mechanisms.
This goal of mechanistic reductionism has been one impetus behind psychometric and cognitive research linking gF with working memory (WM) ability. Namely, WM ability is specified more narrowly than gF, placing it at a level that is more tractable in terms of psychological processes. WM refers to a limited cognitive system involved in the temporary storage and manipulation of information required for task-relevant performance. Numerous factor analytic studies have demonstrated a strong association between individual differences in WM ability and gF (Ackerman, Beier, & Boyle, 2002; Colom, Flores-Mendoza, & Rebollo, 2003; Colom, Rebollo, Palacios, Juan-Espinosa, & Kyllonen, 2004; Conway, Cowan, Bunting, Therriault, & Minkoff, 2002; Engle, Tuholski, Laughlin, & Conway, 1999; Kane et al., 2004; Kyllonen & Christal, 1990; Suess, Oberauer, Wittman, Wilhelm, & Schulze, 2002), with path coefficients between the latent variables ranging from .60 to .90.
This reductionistic perspective can potentially extend from the cognitive to neural level of analysis, by further decomposing constructs. In particular, the cognitive construct of WM can likely be decomposed into domain-specific mechanisms that govern the active maintenance of information over short periods of time, and domain-general central executive processes that regulate and coordinate those maintenance operations (Baddeley, 2007; Osaka, Logie, & D’Esposito, 2007). Cognitive neuroscience research has suggested that these subsystems may be readily mapped onto neural substrates and mechanisms. Thus, inter-individual variability at the level of neural mechanisms might be more strongly tied to these specific subcomponents than to the broader construct of WM as a whole. Therefore, we postulate that theoretical understanding of the broader constructs of gF and WM span may benefit from the investigation of specific subcomponents that relate to both behavioral performance and neural function in a more tractable fashion. It is important to note that this reductionist strategy may also benefit applied research, such as in efforts to enhance intelligence (Buschkuehl & Jaeggi, 2010). Specifically, identifying the core neural and psychological mechanisms that drive variation in gF may provide better targets for training interventions, as well as metrics for evaluating their success.
The goal of the current study was to determine whether the relationship between gF and WM might be mediated by a particular executive subcomponent – interference control – geared towards managing the disruptive effects of irrelevant information on the active maintenance of task goals. We used functional neuroimaging methods to measure the activation of interference control mechanisms, and then directly relate this to shared variance between gF and WM span, as measured by separate psychometric assessments. Finally, we conducted path analyses to determine whether the influence of neural mechanisms related to interference control on gF might result from their effects on individual differences in WM span.
Relating interference control to gF and WM span
The construct of interference control seems a good starting point for understanding the nature of the gF – WM relationship, given suggestive evidence linking interference control with gF and WM span (Bunting, 2006; Dempster & Corkill, 1999; Lustig, May, & Hasher, 2001; May, Hasher, & Kane, 1999). Individual differences in WM ability are typically discussed in terms of “capacity” or “span” – that is, the number of items that can be actively maintained. Strikingly, however, previous studies have demonstrated that the predictive validity of WM span measures depends strongly upon the degree of proactive interference (PI) present in the administration of those tasks. For instance, when the presence of PI is reduced in WM span tasks, there is a strong reduction in the relationship of those modified WM span tasks with age-related cognitive deficits (May et al., 1999) and independent measures of cognitive ability, such as sentence comprehension (Lustig et al., 2001) and gF measures (Bunting, 2006). Because WM span tasks are sensitive to proactive interference, it is highly likely that individual differences in WM span are related, at least in part, to the ability to control interference. Similarly, gF-loaded measures are correlated with performance on interference-sensitive tasks – such as the color-word Stroop test and the Brown-Peterson task – in college-aged adults and elementary school students (Dempster & Corkill, 1999). Also, in a WM span task that included a PI-release condition, performance on high PI trials was more correlated with gF measures than was performance on low PI trials (Bunting, 2006). Altogether, this evidence suggests that the typically observed relationship between gF and WM span reflects, in part, the ability to control interference.
Common neural substrates related to gF, WM span, and interference control?
Evidence supporting the relationship among gF, WM span, and interference control comes from cognitive neuroscience research, which has suggested that these constructs all relate to the structure and function of specific portions of prefrontal cortex (PFC) and parietal lobe (Braver, Gray, & Burgess, 2007; Jung & Haier, 2007; Kane & Engle, 2002). Areas frequently implicated include: A) dorsolateral PFC, including regions of middle frontal gyrus (MFG) around Brodmann Areas (BAs) 9 and 46, B) ventrolateral PFC, including portions of inferior frontal gyrus (IFG) around BAs 45 and 47, C) anterior PFC, including MFG (BA 10 and portions of BA 46) and IFG (portions of BA 47), D) medial PFC, including dorsal anterior cingulate cortex (ACC; BAs 24, 32) and dorsal medial frontal gyrus (BA6; also called pre-supplementary motor area or pre-SMA), E) lateral parietal lobe including inferior parietal lobule (BA40); and F) medial parietal regions, including the precuneus (BA7).
With regard to gF, an early study using fMRI (Prabhakaran, Smith, Desmond, Glover, & Gabrieli, 1997) found that an analytic reasoning task (similar to those used in gF tests) activated a widespread set of brain regions to a greater degree than simple pattern matching. These regions included both dorsolateral and ventrolateral PFC (BAs 6, 9, 44, 45, 46) and medial and lateral parietal cortex (BAs 7, 39, 40), along with temporal (BAs 21, 37) and occipital (BAs 18, 19) cortices. A subsequent PET study (Duncan et al., 2000) found increased activation of bilateral ventrolateral (BAs 45, 47), dorsolateral (BAs 6, 46), and left anterior (BA 10/46) PFC for tasks that correlated more highly with gF relative to matched tasks that correlated more weakly with gF. Another study that performed imaging during a fluid analogies task (Geake & Hansen, 2005) found activation throughout anterior (BA 10), ventrolateral (BAs 45, 47), and dorsolateral (BAs 44, 46) PFC and lateral parietal lobe (BAs 7, 40), but verbal intelligence was correlated specifically with activation within dorsolateral PFC.
A number of studies have demonstrated that individual differences in general intelligence, IQ, or gF are related to the degree of activation in bilateral PFC and parietal cortices, although the direction of the relationship has differed among studies. In a study involving performance of gF-loaded tasks (Lee et al., 2006), gifted individuals showed greater activation in medial (BA 32), dorsolateral (BAs 6, 8, 9, 46), and ventrolateral (BA 45) PFC, as well as parietal (BAs 7, 39, 40) and occipital (BA 19) cortices. In a verb generation task (Schmithorst & Holland, 2006), intellectual performance (IQ) was associated with activity in left ventrolateral PFC (BAs 6, 44, 45), medial PFC (BAs 24, 32), medial parietal (BA 19), and bilateral temporal lobes (BAs 21, 22). In contrast to the studies mentioned above, several studies using PET or EEG have suggested a relationship between increased intelligence and decreased activation (i.e., increased neural efficiency; Haier, Siegel, Tang, Abel, & Buchsbaum, 1992; Haier et al., 1988; Neubauer & Fink 2003, 2009; Neubauer, Fink, & Schrausser, 2002; Neubauer, Grabner, Freudenthaler, Beckmann, & Guthke, 2004). In addition to activation measures, anatomically-based studies have shown that IQ correlates with the volume of gray matter in anterior and dorsolateral PFC (BAs 9, 10, 46), temporal (BAs 21, 22, 37, 42), lateral parietal (BAs 3, 43), and occipital (BA 19) regions (Haier, Jung, Yeo, Head, & Alkire, 2004). Furthermore, variance related to general intelligence accounts for many of the IQ correlations with gray matter in medial PFC (ACC; BA 24), lateral PFC (BAs 8, 10, 11, 46, 47), lateral parietal (BAs 7, 40), temporal (BAs 13, 20, 21, 37, 41), and occipital (BAs 17, 18, 19) cortices (Colom, Jung, & Haier, 2006). Thus, although many studies of gF and intelligence find widespread neural correlates, the most consistent findings relate to the engagement of PFC and parietal cortex.
There has also been extensive study of brain regions involved in WM tasks. A meta-analysis of WM studies (Wager & Smith, 2003) has demonstrated the consistent involvement of a broad set of regions, including dorsolateral, ventrolateral and anterior PFC (BAs 6, 8, 9, 10, 47), lateral and medial parietal cortex (BA 7, 40), and medial PFC (BAs 6, 8, 32). Another meta-analysis focusing specifically on the n-back WM task (Owen, McMillan, Laird, & Bullmore, 2005), found very similar regions (i.e., dorsolateral, ventrolateral, and anterior PFC, medial and lateral parietal cortex, plus medial PFC) engaged consistently across studies. Studies of WM employing complex span tasks similar to those typically used in the psychometric and individual differences literature have also implicated a highly consistent set of brain regions. Bunge and colleagues (Bunge, Klingberg, Jacobsen, & Gabrieli, 2000) and Smith and colleagues (Smith et al., 2001) found increased lateral PFC and parietal activation in dual-task WM tasks (analogous to complex span tasks). A recent study (Chein, Moore, & Conway, 2011) specifically investigating the presence of domain-general processes during verbal and spatial span tasks found activation within dorsolateral PFC, medial PFC and lateral parietal cortex. A small number of studies have also investigated how individual differences in WM span impact brain structure and function. During performance of a complex span task, high span subjects showed increased activation was observed in ventrolateral, dorsolateral, and medial PFC, as well as parietal cortex, relative to low span subjects (Kondo, Morishita, Osaka, & Osaka, 2004; Osaka et al., 2003, 2004; Osaka, Komori, Morishita, & Osaka, 2007). Furthermore, some evidence (Colom, Jung, & Haier, 2007) has tied individual differences in WM span, gF, and gC to gray matter volume in left parietal lobe (BAs 7, 40) as well as bilateral regions of ventrolateral and anterior PFC (BAs 10, 47).
As with gF and WM span, interference control has also been associated with the function of lateral PFC, medial PFC, and parietal lobe. Several studies have investigated the specific role of left ventrolateral PFC (BAs 45, 47) in the recent negatives task, a paradigm modified from the classic Sternberg WM task (Sternberg, 1966) to examine interference control during WM (D’Esposito, Postle, Jonides, & Smith, 1999; Jonides, Smith, Marshuetz, Koeppe, & Reuter-Lorenz, 1998). In this task, interference occurs on recent negative trials, in which the probe item was absent from the memory set on the current trial, but was present in the memory set on the previous trial. Individuals with lesions in left ventrolateral PFC experience increased interference in the recent negatives task (Hamilton & Martin, 2005; Thompson-Schill et al., 2002). Other studies using the recent negatives paradigm (Badre & Wagner, 2005; Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001; Burgess & Braver, 2010; Mecklinger, Weber, Gunter, & Engle, 2003; Nelson, Reuter-Lorenz, Sylvester, Jonides, & Smith, 2003) have implicated additional regions in interference control, including anterior (BA 10), dorsolateral (BA 9), and medial PFC (BAs 6, 32), lateral and medial parietal lobe (BA 7, 40) and premotor cortex (BA 6). A meta-analysis (Nee, Wager, & Jonides, 2007) of neuroimaging studies involving a wider range of standard interference task paradigms (i.e., Stroop, Flanker, go/no-go, stimulus-response compatibility, and Simon) suggest that these same regions are consistently engaged when control over interference is required. Interestingly, correlations between activation of lateral PFC regions and behavioral interference have not been entirely consistent, with some results showing negative correlations between activation and interference (Bunge et al., 2001) but others showing positive correlations (Badre & Wagner, 2005).
Several studies have examined how individual differences in WM span relate to the neural systems engaged during interference control. Compared to low WM span subjects, high WM span subjects have shown reduced interference-related activation across several regions, including a posterior lateral PFC region sometimes termed inferior frontal junction (IFJ) around the junction of MFG and precentral gyrus (BAs 6, 9, and 44), along with medial and lateral parietal cortex (Mecklinger et al., 2003). During a spatial WM task, higher WM capacity was related to increased activity in dorsolateral PFC and basal ganglia regions when there was an expectation to filter task-irrelevant information (McNab & Klingberg, 2008). Edin and colleagues (2009) developed a computational model in which parietal cortex was involved in short-term storage of item information, but was subject to interference between representations due to lateral inhibition. The model included a mechanism that provides a top-down excitatory bias to boost WM capacity selectively under high-load conditions, by transiently overcoming this lateral inhibition. The pattern of activation predicted by the top-down bias mechanism was detected in dorsolateral PFC (BA 9, 46), supporting the idea that increased WM capacity in some individuals results from the ability to engage this region to overcome perceptually-related interference during short-term storage. Supporting this general conceptualization, another recent study found that, compared to individuals with low WM span, those with high WM span showed greater activation of left dorsolateral PFC and less activation of fusiform face area during a high-distraction version of a face working memory task (Minamoto, Osaka, & Osaka 2010).
Two specific studies are notable for investigating the relationship between neural mechanisms of interference control and gF. Gray and colleagues (Gray, Chabris, & Braver, 2003) conducted a key study demonstrating that the shared variance in gF and interference control ability was related to brain activity in lateral PFC and parietal cortex. Interference control was examined within the n-back task. The n-back task is typically thought to require the updating of information in WM, because, for each sequentially presented item, the participant must judge whether it matches the item presented n trials back (where n is prespecified, and usually equals 1, 2, or 3 items). However, the n-back task can also be used to examine interference control, by focusing on lure trials, which match a recently presented item but do not match the target item (e.g., 2, 4, or 5-back repeats in a 3-back task). Lure trials are subject to interference due to a strong familiarity signal that could lead to an incorrect target response. It was found that individual differences in gF related to both lure accuracy and the event-related fMRI response on lure trials in dorsolateral PFC (BAs 9, 44, 46), medial PFC (ACC; BA 24), lateral parietal (BA 40), and bilateral temporal (BA 22) regions. Furthermore, the relationship between gF and accuracy on lure trials was statistically mediated by activity in dorsolateral PFC and parietal regions.
More recently, Burgess & Braver (2010) investigated the role of interference expectancy on the temporal dynamics of brain activity during interference control, and the modulatory role of gF. Interference expectancy was manipulated in the recent negatives task, by varying the ratio of recent negative probes to recent positive probes (i.e., present in both the previous and current memory sets) across task blocks. During low expectancy blocks, brain activity related to interference control was seen specifically in response to the recent negative probe, in terms of increased activation in the canonical left ventrolateral PFC region (BAs 44, 45, 47) seen in prior studies, along with medial PFC (ACC/pre-SMA; BA6, 32), and lateral parietal cortex (BA 40). However, during high expectancy blocks, there was a shift in the temporal dynamics of interference control such that activation was seen proactively (i.e., during the delay period before the onset of the probe) within adjacent portions of the same brain regions (i.e., ventrolateral, dorsolateral, and medial PFC). Moreover, it was found that this proactive pattern of activity dynamics was more prominent in the high gF individuals, particularly within dorsolateral (BA 9) and medial PFC (BA 6).
Current Study: Directly testing the role of interference control in WM-gF relationship
Altogether, the literature reviewed above strongly supports the idea that a core component of variability in gF and WM span is the effectiveness of interference control mechanisms in lateral PFC and parietal cortex. However, there has not yet been a direct investigation of the tripartite relationship between interference control, WM span, and gF. In particular, a key unanswered question is whether neural mechanisms of interference control are the common construct underlying the shared variance between WM span and gF. Direct evidence supporting this hypothesis would highlight the central importance of interference control mechanisms in understanding cognitive individual differences. Although the prior work, particularly the studies of Gray et al (2003) and Burgess & Braver (2010), has investigated interference control within the context of WM task performance, those findings do not allow strong and independent claims regarding individual differences in WM ability. This is because the WM tasks that were employed in these prior studies are fairly dissimilar from the complex span-type tasks commonly used and robustly validated in the psychometric and individual differences literature. For example, some studies have shown only weak associations between performance on the n-back and standard WM span tasks (Conway et al., 2005; Kane, Conway, Miura, & Colflesh, 2007). Moreover, in our opinion, the most productive research strategy may be to assess both WM span and gF, using standard psychometric tests administered “out-of-scanner”, and then relate these to an independent, “in-scanner” assessment of interference control during WM task performance.
Based on this experimental logic, the current study aimed to directly and comprehensively quantify the degree to which both neural and behavioral measures of interference control could account for the relationship between gF and WM span. To accomplish this, we collected independent neuroimaging and psychometric data on a relatively large (for neuroimaging studies) sample of ~100 individuals. Specifically, for each individual we collected out-of-scanner measurements of WM span and gF, using two spatial and two verbal WM span tasks, and two frequently utilized gF measures. Additionally, we collected neuroimaging and behavioral data related to interference control using event-related fMRI scanning during performance of the n-back WM task, focusing specifically on high-interference lure trial activity within the 3-back condition. It is worth noting that the choice of lure trials in the n-back task as the neural and behavioral measure of interference control was made for a couple of reasons. First, as mentioned previously, the n-back task is probably the most widely used and well-validated probe of WM-related brain activation within the neuroimaging literature (Owen et al., 2005), including prior studies from our own lab (Braver et al., 1997; Braver et al., 2001). Second, in our prior research (Gray, Chabris, & Braver, 2003), we demonstrated that lure trial activity in the n-back task and performance is strongly predicted by gF. Thus, the current study represents a natural follow-up and extension of the prior work, by testing whether the prior findings replicate in an independent, larger, and more comprehensive dataset that includes additional measures of WM span.
The key prediction of the study is that interference control ability, as reflected in n-back lure trial accuracy, could explain a significant portion of the variance shared between gF and WM span. More importantly, we predicted that interference control ability would be reflected at the neural level in terms of variability in lure-related activity within the specific set of brain regions postulated to subserve interference control (e.g., dorsolateral PFC, ventrolateral PFC, medial PFC, and lateral parietal cortex). Furthermore, using variance partitioning and path analysis, we sought to estimate quantitatively the degree to which these interference control mechanisms capture individual differences in gF through their influence on the psychological construct of WM span.
Methods
The data presented here were collected as part of a larger, multi-faceted study. Individuals participated in three separate sessions, spaced a few days to a few weeks apart, to complete personality tests, mood questionnaires, neuropsychological tests, and the n-back fMRI scanner task. The first session was 3 hours long and involved answering several standard paper-and-pencil questionnaires; the second was 2 hours long and involved answering computerized questionnaires and cognitive tasks; the third was 2.5 hours long, and involved a fMRI scan of the n-back task. Data from these participants have been used in other manuscripts to address questions distinct from those in the current study (DeYoung et al., 2010; DeYoung, Shamosh, Green, Braver, & Gray, 2009; Fales et al., 2008; Shamosh et al., 2008; Welborn et al., 2009; Yarkoni, Barch, Gray, Conturo, & Braver, 2009).
Participants
A total of 121 adult participants were recruited from student and community participant pools at Washington University in St. Louis. Participants received $25 per hour for participation in the MRI session, and $10 per hour (or research credit for undergraduate participants) for participation in tasks outside of the scanner. Participants were all right-handed, native English speakers, with no known neurological or psychiatric disorders. All participants had normal or corrected-to-normal vision. Participants gave informed consent in accordance with guidelines established by Washington University Medical Center and Hilltop Human Studies Committees.
Subsequently, 19 subjects were dropped due to technical or data quality problems (e.g., incomplete scans, excessive motion, chance-level performance), resulting in a final count of 102 participants (58 female, age: mean = 22.21, sd = 4.78, range = 18 – 39). Because there were nine separate behavioral measures of interest (two gF measures, four WM span measures, and three n-back trial type accuracy measures), typical methods of excluding outliers based on their performance on individual measures would have been too stringent, resulting in the exclusion of a large proportion of the subjects (assuming independence of these measures). Therefore, a multivariate approach using the Mahalanobis Distance (Lattin, Carroll, & Green, 2003) was used to identify outliers within this group. This approach identified two potential outliers, comprising roughly 2% of the total number of participants. Analyses conducted with and without these two participants were virtually identical. Therefore, the results reported included these two participants.
gF and WM span factors
gF measures
RAPM
The Raven Advanced Progressive Matrices – Set II is a widely utilized individual difference measure of gF (Raven, Raven, & Court, 1998). The RAPM is a 36-item test of novel problem-solving and reasoning abilities, in which subjects complete the last cell of a 3×3 matrix of figural patterns with one of eight possible completion alternatives. Participants were informed that the items had been arranged in order of increasing difficulty. Participants completed this task in a group administration setting and were allotted 40 minutes to complete the test. The 40 minute time limit was adopted to facilitate group administration of the RAPM task. The RAPM score was taken as the total number of questions answered correctly within 40 minutes (maximum score of 36).
Cattell Culture Fair
The Cattell Culture Fair Test (Cattell, 1973) is a timed test, composed of 4 separate paper-and-pencil subtests. Participants were allotted between 2.5 and 4 minutes for each individual subtest, and were not permitted to continue work on an individual subtest after the allotted time had expired. The first subtest (Series) contained 13 incomplete series of abstract shapes or figures. Participants were to select the best completion for the series from the 6 alternatives provided. The second subtest (Classifications) contained 14 problems. For each problem, there were five abstract figures or shapes presented. Each figure or shape differed from the others in size, orientation, or content. The participant was to choose the two shapes that differed from the other three shapes. The third subtest (Matrices) was similar to the RAPM. Participants were presented with 13 incomplete matrices (four to nine block grids with one empty block). Their task was to choose the one of the six choices provided which best fit in the empty space, based on the relationships among the items in the matrix. In the fourth subtest (Conditions), there were 10 problems, each consisting of single dot placed in relationship to a set of lines and figures. The participant had to choose the one alternative that had the same relationship between the dot and the figures and lines as the exemplar. The final score on the Cattell test was the sum of the correct answers across all four subtests (maximum score of 50).
WM Span Tasks
Operation Span
Participants were required to solve a series of mathematical equations while simultaneously remembering a list of unrelated words. A series of displays was presented, with each display consisting of a math problem and a word (e.g., “IS 3 × 2 + 2 = 7? WALL”). The participant read the math problem aloud, answered “yes” or “no” to whether the given answer was right or wrong, and then read the word. The answer to the math problem was correct on 50% of the displays. After the participant read the word, the next display was presented. After a certain number of displays were presented, three question marks (“???”) were presented to prompt the participant to recall all of the words presented in the series. The number of displays in each series varied between two and five. Three series of each length were performed (total of 12 series). The order of presentation of the series was randomized, such that participants didn’t know the length of a particular series until the retrieval prompt appeared.
Reading Span
Participants read a series of sentences aloud while simultaneously remembering a list of letters. A series of displays was presented, with each display consisting of a sentence 12–14 words long, followed by a capital letter. The participant read the sentence aloud and then read the capitalized letter. After the participant read the capital letter, the next display was presented. After a certain number of displays were presented, three question marks (“???”) were presented to prompt the participant to recall all of the capital letters presented in the series. The number of displays in each series varied between two and five. Three series of each length were performed (total of 12 series). The order of presentation of the series was randomized, such that participants didn’t know the length of a particular series until the retrieval prompt appeared.
Rotation Span
In the rotation span task, participants saw a series of displays, each of which consisted of the image of a capital letter, rotated by some number of degrees (in multiples of 45°). The letter was either normal or mirror imaged, and the participant’s task was to state whether the letter was normal or mirror imaged. After responding, an arrow appeared, either short or long in length, and rotated by some number of degrees (in multiples of 45°). The participant was required to remember the location to which the arrow was pointing (16 potential locations). After each series, the participants recalled the order of locations in which the arrows were presented on an answer sheet containing a figure of the sixteen possible locations. The number of displays in each series varied between two and five. Three series of each length were performed (total of 12 series). The order of presentation of the series was randomized, such that participants didn’t know the length of a particular series until the series ended.
Symmetry Span
Participants received a series of displays, consisting of an 8 × 8 black-and-white grid. They were required to state whether or not the display was vertically symmetrical. After responding, a second display was presented containing a 4 × 4 grid with one red square. After a certain number of displays were presented, three question marks (“???”) were presented to prompt the participant to recall the order and locations of the red squares presented in the series. The number of displays in each series varied between two and five. Three series of each length were performed (total of 12 series). The order of presentation of the series was randomized, such that participants didn’t know the length of a particular series until the retrieval prompt appeared.
The span scores were computed identically for all four span tasks. A partial-credit, unit-weighted scoring method was utilized, because it was previously demonstrated to yield higher internal consistency and more normally distributed scores than other scoring procedures (Conway et al., 2005). Unlike full credit scoring methods, correctly recalled items were given partial credit, even if no other items were recalled correctly within a list (e.g., if only one item was recalled from a list of five items, that correct item was still counted toward the score). Recalled items were only considered correct if they were listed in the correct serial position. In addition, rather than being weighted by the number of units within a list, lists of various lengths were weighted equally, such that perfect performance on a list of three items affected the score as much as perfect performance on a list of five items. The proportion of correct responses was computed separately for each list (i.e., 3 out of 5 = 0.6, 2 out of 2 = 1.0). Then, these proportion scores were then averaged across all of the lists to yield participant’s task score. Scores therefore could range from 0 to 1.
Factor scores
Factors were created independently for gF and WM span from the individual measures. Two principal components analyses (PCA) with promax rotation were conducted to derive a WM span factor from the four WM span measures, and gF factor from the two gF measures. Only factors with eigenvalues greater than 1 were retained. One WM span factor was identified from the four individual WM measures, accounting for 68.4% of the overall variance. One gF factor was identified from the two gF measures, accounting for 87.1% of the overall variance.
N-back Task
During fMRI scanning, participants engaged in a 3-back version of the n-back task, an extensively-used paradigm in the neuroimaging literature (see Owen et al., 2005 for a meta-analysis of n-back neuroimaging studies) typically assumed to reflect WM and/or executive function (but see Kane et al., 2007). During task blocks, a continuous series of stimuli – words in some blocks, faces in other blocks – were presented individually and sequentially on a screen for 2 sec each, followed by a 360-msec fixation cross. For each stimulus, participants were instructed to press one of two buttons to indicate if the current item was identical to the item presented three trials previously (i.e., target trials), or different from the item presented three previously (i.e., nontarget trials). Null fixation trials, 2.36 sec in duration, were randomly interspersed in the sequence of trials to add temporal “jitter” to the timecourse of events. Furthermore, during scanning, blocks of task were preceded and followed by blocks of passive rest lasting 35.4 seconds.
Before entering the scanner, participants were given instructions and practice at the three-back task. The practice trials were repeated if necessary. The scanning session included six scan runs of the n-back task, each immediately preceded by a mood-inducing video. The videos included approximately 10 min each of passive viewing that was intended to induce either a positive, negative, or neutral mood state. The order of mood-induction videos was counterbalanced across participants. Scanning was not conducted during video presentation. For the present study, we only analyzed data from the two scans (one face, one word) that were preceded by the neutral-mood video.
During analysis, nontarget trials were divided into two separate types. Lure nontargets had been presented previously in the task block, but not in the 3-back position, and nonlure nontargets had never been presented prior to the current trial. It is noteworthy to mention that the definition of lure nontargets was designed in the current study to be inclusive of any familiar nontarget stimulus. More specifically, the study by Gray and colleagues (2003) defined lures as only 2, 4, or 5-back repeated nontargets. However, in the current study, all repeated stimuli that were not 3-back targets were classified as lures, and all nonrepeated stimuli were classified as nonlures. Across the 128 task trials (64 word trials and 64 face trials), there were 40 targets (31.25% of trials), 24 lures (18.75% of trials), and 64 nonlures (50% of trials). Accuracy for each trial type was calculated as the proportion of correct responses relative to the number of overall responses for that trial type.
Materials
The 3-back task was administered on an Apple Power Macintosh G3 computer, using Psyscope (Cohen, MacWhinney, Flatt, & Provost, 1993) experimental software for stimulus presentation and response collection. Stimuli were presented via a LCD projector onto a Lucite screen, and were viewed by the subject via a mirror fixed on the scanner head coil. Subjects responded to the task stimuli by pressing one of two buttons on a fiber-optic button box held in their right hand. This button box was capable of capturing responses and response latencies with millisecond accuracy.
The face run consisted of a mixture of unfamiliar male and female faces (Kelley et al., 1998). The word run consisted of concrete English nouns of 1 or 2 syllables (mean = 1.46, sd = 0.50), with a mean frequency of 60.45 uses per million (Kucera & Francis, 1967) and a mean length of 5.07 letters (sd = 0.87). Both face and word stimuli were emotionally neutral. The order of stimulus lists was counterbalanced across participants.
Scanning Procedure
In order to estimate sustained and transient activation effects separately, we used a mixed design (Donaldson, Petersen, Ollinger, & Buckner, 2001; Visscher et al., 2003). A mixed design contains blocks of task trials that alternate with blocks of rest. Also, within each task block, trials are presented with variable-length fixation trials randomly interspersed between trials. Each scan run lasted for 361.08s (153 volumes at 2.36s per volume), and was comprised of two 3-back task blocks and three blocks of passive rest (i.e., null fixation trials). The first four volumes of each run were deleted to allow steady-state magnetization. Each task block contained 52 volumes: 32 trial volumes and 20 randomly intermixed “jittered” fixations (“+”). The fixation blocks each contained 15 frames, with a “−” presented in the center of the screen for each frame during the fixation block.
fMRI Parameters
fMRI images were collected on a Siemens 3T Allegra scanner (Erlangen, Germany) with a standard circularly polarized head coil. High-resolution structural scans were acquired prior to the functional sequences using a sagittal MP-RAGE T1-weighted sequence (1 × 1mm in-plane resolution, 1.25 mm slices). Functional whole-brain images were collected using an asymmetric spin-echo echo-planar sequence sensitive to BOLD contrast (T2*: TR = 2.36 s, TE=25 ms, flip angle=90°, field of view=256mm, 4 mm × 4 mm in-plane resolution, 64×64 matrix, 32 contiguous slices; slice width 4mm).
fMRI data analysis
Prior to statistical analysis, functional volumes were corrected for slice-timing asynchrony within each volume, normalized to a fixed intensity value to reduce across-run effects of scanner drift and scanner instability, motion corrected using a rigid-body transformation (Friston, Williams, Howard, Frackowiak, & Turner, 1996; Snyder, 1996), and registered to the high-resolution anatomical image. The functional data were then resampled into 3mm isotropic voxel dimensions and spatially smoothed with a Gaussian kernel (FWHM = 9mm). Finally, volumes were transformed to a standardized atlas target in Talairach coordinate space (Talairach & Tournoux, 1988) using a 12-dimensional linear affine transformation (Woods, Grafton, Holmes, Cherry, & Mazziotta, 1998).
Independent parameter estimates were computed using a general linear model (GLM) with both sustained and event-related regressors (Donaldson et al., 2001). The sustained regressors were boxcars convolved with a gamma function that coded for sustained responses across each type of task block (i.e., face block, word block). Regressors for each of the three trial types (targets, “lures,” and “nonlures”) were coded by a series of eight delta-function regressors, starting at the trial onset and lasting for a total of 18.88 sec, allowing for the independent estimation of the hemodynamic response for each trial type. Only correct trials were included in these event-related regressors. Magnitude estimates of the activity for event-related responses were defined as the cross-correlation of the estimated BOLD response over the eight timepoints with a canonical hemodynamic response function, modeled as a gamma function with a delay of 2 sec and a time constant of 1.25 sec (Boynton, Engel, Glover, & Heeger, 1996). The sustained activity effect can be conceptualized as the magnitude of sustained activity during task blocks relative to resting fixation blocks. The transient/event-related effects can be conceptualized as a contrast between activity for a specific trial type and activity during within-block fixations. In order to investigate the neural substrates of interference control, we focused subsequent analyses on event-related activation related to lure trials and on sustained activation that was correlated with block-wise lure accuracy.
Group whole-brain analyses were conducted to identify regions that showed activation significant at a multiple-comparison corrected threshold of p < .05 (z > 3.25, clusters of 25 or more contiguous voxels, corrected using Monte Carlo simulation (Forman et al., 1995). Subsequent conjunction analyses identified subsets of these voxels that also showed correlations between activity and each of the following variables: lure accuracy, gF factor, and WM span factor (voxelwise p < .05, ROIs composed of 16 or more contiguous voxels).
Variance partitioning
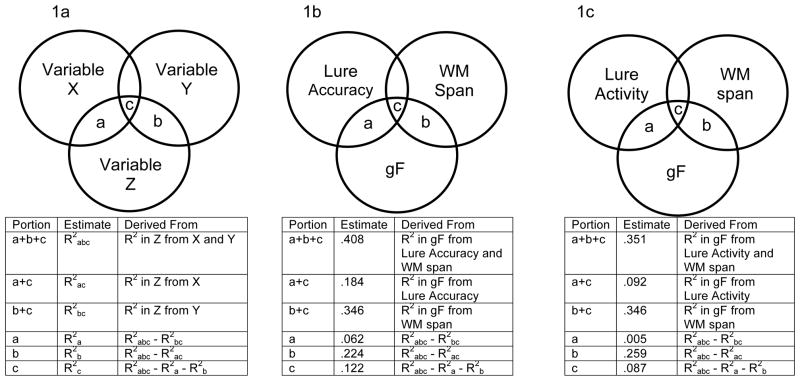
To determine the portion of shared variance in gF and WM span that was related to interference control, we used a variance-partitioning method (cf., Chuah & Maybery, 1999; Salthouse, 1994). To estimate the portions of variance in a criterion variable Z that are shared with and unique to two predictors X and Y, one begins by estimating three regression equations: first, R2 in Z from X and Y; second, R2 in Z from X; and third, R2 in Z from Y. The unique portions of variance can be derived by subtracting the variance explained by either the second or third equations from the first equation. For instance (see Figure 1a), variance portion b can be calculated by subtracting the second equation from the first equation. Subsequently, the shared portion can then be calculated by subtracting the unique portions from the overall explained variance in the first equation (i.e., c equals a+b+c minus a+b). The proportion of the shared variance between Z (gF) and Y (WM span) that is common to X (interference control) can be calculated as c/(b+c).
Figure 1.
Venn diagrams illustrating variance-partitioning method used to divide variance in gF, WM span, and interference control measures into common and shared portions. Figure 1a illustrates the general method conceptually. Figure 1b shows the variance portions related to lure accuracy. Figure 1c shows the variance portions related to lure activity factor scores. N.B., size of the overlapping circles is not drawn in proportion to the amount of variance explained by those portions.
Path analysis
The path model had two main features: first, lure-related activity was free to influence gF through a direct pathway or through an indirect pathway via lure accuracy and WM span; and second, lure accuracy was free to influence gF through a direct pathway and an indirect pathway through WM span. The significance of the indirect paths was tested using a bootstrap method (Shrout & Bolger, 2002; bias-corrected confidence interval method, 2000 bootstrap samples). It is noteworthy that the path model is not only consistent with literature relating these constructs, but also with a reductionist approach of explaining more general and behaviorally measured constructs with more specific and neurologically tractable constructs.
Results
Behavioral results
Table 1 reports the descriptive statistics for all of the individual n-back, gF, and WM span measures. The zero-order correlations among the n-back accuracy measures and the individual gF and WM span tasks are reported in Table 2. Table 3 shows the relationships among the gF and WM span factor scores and the n-back accuracy measures.
Table 1.
Descriptive Statistics for n-back, gF, and WM Span Measures
| Mean | Std. Error of Mean | Standard Deviation | Minimum | Maximum | |
|---|---|---|---|---|---|
| RAPM | 25.41 | .59 | 5.99 | 7 | 35 |
| Cattell | 29.81 | .51 | 5.12 | 13 | 38 |
|
| |||||
| OSPAN | .7557 | .0129 | .1302 | .238 | 1.000 |
| READSPAN | .8197 | .0129 | .1306 | .206 | 1.000 |
| ROTSPAN | .6829 | .0134 | .1358 | .264 | 1.000 |
| SYMSPAN | .6126 | .0195 | .1968 | .100 | 1.000 |
|
| |||||
| Lure Acc | .7365 | .0166 | .1681 | .23 | 1.00 |
| Target Acc | .7703 | .0122 | .1228 | .43 | 1.00 |
| Nonlure Acc | .9817 | .0025 | .0254 | .86 | 1.00 |
Note: RAPM = Raven’s Advanced Progressive Matrices
Table 2.
Zero-order Correlations Among Individual gF, WM Span, & n-back Accuracy Measures
| RAPM | Cattell | OSPAN | READSPAN | SYMSPAN | ROTSPAN | Lure Acc | Target Acc | Nonlure Acc | |
|---|---|---|---|---|---|---|---|---|---|
| RAPM | .742** | .412** | .327** | .427** | .461** | .420** | .301* | .412** | |
| Cattell | .742** | .532** | .371** | .588** | .464** | .380** | .407** | .313** | |
| OSPAN | .412** | .532** | .638** | .586** | .468** | .299* | .260* | .114 | |
| READSPAN | .327** | .371** | .638** | .563** | .506** | .212* | .371** | -.090 | |
| SYMSPAN | .427** | .588** | .586** | .563** | .706** | .281* | .455** | .315** | |
| ROTSPAN | .461** | .464** | .468** | .506** | .706** | .306* | .435** | .306* | |
| Lure Acc. | .420** | .380** | .299* | .212* | .281* | .306* | .270* | .279* | |
| Target Acc. | .301* | .407** | .260* | .371** | .455** | .435** | .270* | .026 | |
| Nonlure Acc. | .412** | .313** | .114 | -.090 | .315** | .306* | .279* | .026 |
p < .05;
p < .001
Table 3.
Zero-order Correlations Between gF Factor, WM Span Factor, & n-back Accuracy
| gF factor | WM span factor | Lure Accuracy | Target Accuracy | Nonlure Accuracy | |
|---|---|---|---|---|---|
| gF factor | .589** | .429** | .379** | .389** | |
| WM span factor | .589** | .332** | .462** | .199* | |
| Lure accuracy | .429** | .332** | .270* | .279* | |
| Target accuracy | .379** | .462** | .270* | .026 | |
| Nonlure accuracy | .389** | .199* | .279* | .026 |
p < .05;
p < .001
The relationships between the individual measures of gF and WM span (Table 2) were weaker than the relationship between the gF and WM factor scores (Table 3). Similarly, the correlations of n-back accuracy with gF and WM span were greater in most cases for the gF and WM factor scores (Table 3) than for individual measures of gF and WM span (Table 2). The magnitude of the correlation between the gF factor and the WM factor was relatively large, r(100) = .59 (Table 3) and was roughly within the range of effect sizes reported previously in the literature (Ackerman et al., 2002; Colom et al., 2003; Colom et al., 2004; Conway et al., 2002; Engle et al., 1999; Kane et al., 2004; Kyllonen & Christal, 1990; Suess et al., 2002). This demonstrates that there is a substantial relationship between these two constructs, and suggests the presence of shared processes that influence individual differences in performance.
To determine whether a gF factor derived from the RAPM and Cattell measures might relate differentially to spatial WM span versus verbal WM span, separate measures of spatial and verbal WM span were derived using principal component analysis. The correlation between spatial WM span and the gF factor was r (100) = .57, p < .001, whereas the gF – verbal WM span correlation was r (100) = .49, p < .001. The spatial WM span factor explained unique variance in gF above and beyond verbal WM span, R2change = .12, F(1, 99) = 18.32, p < .001. Therefore, it seems that the gF – WM span correlation is somewhat stronger for spatial measures than for verbal measures. However, the WM span factor derived from all four WM span measures still relates to gF quite strongly, r (100) = .59 (Table 3), in comparison to the spatial or verbal WM span factors alone. This pattern suggests that, compared to performance on the individual measures of gF and WM span, the gF and WM factor scores were influenced more by variance related to the general constructs and less by method variance and statistical error. Furthermore, previous research shows that domain-general variance in WM span measures is more strongly related to gF than is domain-specific variance (Kane et al., 2004). For these reasons, all subsequent analyses were conducted on the factor scores for gF and WM span.
Based on the premise that WM span and gF were related to interference control ability, we expected to find that both of these factors related to lure accuracy, above and beyond the performance on low interference n-back trials. As expected, the correlations between the 3-back accuracy measures (lures, nonlures, and targets) and the gF and WM span factors were statistically significant (Table 3). The correlation of lure accuracy with gF, r(100) = .43, was still statistically significant after controlling for nonlure accuracy, pr = .362, p < .001, and target accuracy, pr = .366, p < .001. The magnitude of this relationship was similar to that reported by Gray and colleagues (2003). Likewise, the correlations between WM span and lure accuracy, r(100) = .33, also persisted after controlling for nonlure accuracy, pr = .294, p = .003, and target accuracy, pr = .242, p = .015.
As found previously (Gray et al., 2003), mean RT from the n-back task did not correlate with gF factor scores for any of the individual trial types. WM span factor scores correlated with mean RT for correct lure nontargets, r(100) = .20, p = .044, and for correct nonlure nontargets, r(100) = .24, p = .015, but not with mean RT for targets, r(100) = .06. These positive correlations with nontarget RT, combined with those for accuracy, suggest that higher WM span corresponded to slower but more accurate responses to nontarget trials. Importantly, given that mean RT measures correlated with WM span but not with gF, it is not possible that response speed explained any of the gF – WM span common variance. Thus, correlations with RT will not be discussed in subsequent analyses involving 3-back performance.
To determine the portion of shared variance in gF and WM span that was related to lure accuracy, we utilized the variance-partitioning method described in the Methods (see Figure 1b). Both WM span (R2 = .346) and lure accuracy (R2 = .184) captured variance in gF. Of the gF variance related to lure accuracy and WM span, there were both shared and unique influences of those measures. The unique contribution of lure accuracy captured 6.2% of gF and the unique contribution of WM span variance captured 22.4% of gF variance. However, variance common to lure accuracy and WM span captured 12.2% of gF variance. Stated differently, of the variance shared by gF and WM span (b+c in Figure 1b), 35.3% was also related to lure accuracy variance (c in Figure 1b).
Imaging results
To address whether individual differences in the neural processes involved in interference control might participate in the common relationship between gF and WM span, we utilized a logical conjunction approach to restrict regions of interest (ROIs) to those voxels that showed a specific pattern of event-related activity (described in more detail in Methods). First, we identified voxels that showed statistically significant activation for either lure trials or for sustained activity, suggesting that they were actively involved in the performance of the task. To ensure that these voxels were related to interference control, we further constrained the activation maps to those voxels in which activity was correlated with individual differences in lure accuracy, either positively or negatively. Lastly, to narrow the search to those interference control regions that potentially share variance with the gF – WM span relationship, the activation maps were constrained in a final step to those voxels in which activity was also correlated with individual differences in both gF and WM span, either positively or negatively.
Ten ROIs showing a relationship with lure activity were identified based on this analysis (Table 4) that included regions in bilateral dorsolateral PFC (middle frontal gyrus) and parietal cortex (inferior parietal lobule). Importantly, even though these ROIs were defined based on activity specific to lure trials during the 3-back task, they comprise a canonical set of regions that overlaps well with both meta-analyses of the n-back task (which have typically used block-based, rather than trial-type-based analyses; Owen et al., 2005), and more traditional studies of gF and WM tasks (Duncan et al., 2000; Lee et al., 2006; Prabhakaran et al., 1997; Wager & Smith, 2003), while also closely replicating the original findings of Gray et al (2003). Moreover, for all 10 of these regions the correlations between lure trial activity and the individual difference factors were in the positive direction, meaning that increased lure activity was associated with more accurate lure performance, and higher gF and WM span. Although a number of distinct regions showed significant sustained activation, none of these regions also showed consistent correlations between the sustained activity and the conjunction of individual difference factors.
Table 4.
Regions of Interest demonstrating correlations with gF, WM span, and lure activity (see Methods). Partial correlations show the relationship between lure activity and gF or WM span after controlling for target and nonlure activity in that ROI (pr gF and pr WM).
| Regions | BA | Coordinates | mm3 |
r gF |
r WM |
pr gF |
pr WM |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Left Dorsolateral PFC (Middle Frontal Gyrus) | 6/9 | −40 | 9 | 30 | 3321 | .263* | .360** | .283* | .302* |
| Right Dorsolateral PFC (Middle Frontal Gyrus) | 9 | 44 | 18 | 30 | 3591 | .280* | .374** | .310* | .356** |
| Left Precentral Gyrus; Medial PFC (Medial Frontal Gyrus) | 6 | −28 | −3 | 54 | 2349 | .247* | .304* | .324** | .330** |
| Right Superior Frontal Gyrus; Medial PFC (Medial Frontal Gyrus) | 6 6/32 |
20 | 0 | 54 | 7074 | .300* | .378** | .383** | .402** |
| Left Lateral and Medial Parietal (Inferior Parietal Lobule/Precuneus) | 40/7 | −28 | −66 | 42 | 1998 | .222* | .316** | .268* | .353** |
| Right Lateral and Medial Parietal (Inferior Parietal Lobule/Precuneus) | 40/7 | 38 | −57 | 45 | 6831 | .264* | .377** | .319** | .388** |
| Right Medial Parietal (Superior Parietal Lobule) | 7 | 8 | −66 | 48 | 837 | .278* | .335** | .296* | .313* |
| L Fusiform Gyrus | 18 | −22 | −81 | −24 | 459 | .268* | .364** | .290* | .209* |
| L Parahippocampal Gyrus; Thalamus | 27 | −14 | −27 | 3 | 1431 | .242* | .373** | .317** | .354** |
| R Parahippocampal Gyrus; Thalamus | 27 | 20 | −33 | 0 | 324 | .268* | .356** | .324** | .357** |
To determine if the relationship between lure activity and the two individual difference factors was specific to processes exclusive to lure trials, we computed correlations after partialling variance related to target and nonlure trials. Lure activity levels in each of these ROIs were still significant predictors of gF and WM span, even after controlling for target and nonlure activity levels via partial correlation. This fact suggests that the relationship of gF and WM span with lure activity in these ROIs was not affected by individual differences in more general neural processes recruited during target and nonlure trials or sustained across the entire task block. These data support the hypothesis that some portion of variance in gF and WM span is related to interference control.
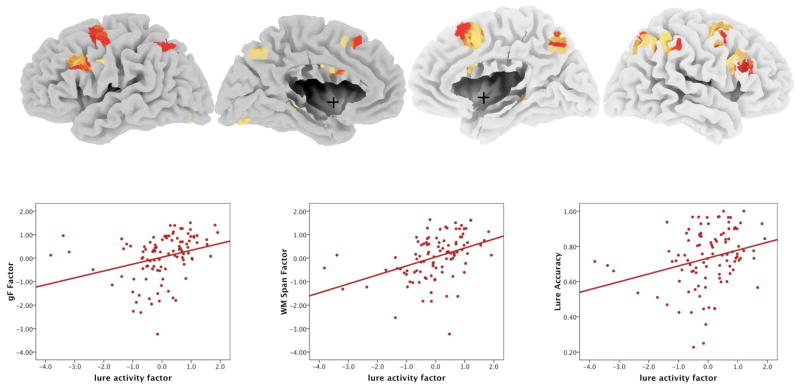
We extracted the parameter estimates for lure-related activity from these 10 ROIs to determine the proportion of gF – WM span common variance that could be explained by interference control mechanisms. The activity estimates from the 10 ROIs had similar correlations with gF, WM span, and lure accuracy. Therefore, we simplified further analyses by reducing the data dimensionality of the activity estimates from these regions. The parameter estimates from the 10 ROIs were entered into a principal components analysis to reduce these variables into orthogonal components. This analysis yielded only one factor with an eigenvalue greater than 1. This factor explained 75.8% of the overall variability in the lure-related parameter estimates from those 10 ROIs. Scatterplots demonstrating the correlations of this lure activity factor with gF, WM span, and lure accuracy are shown in Figure 2.
Figure 2.
Brain regions in which lure-related activity correlates with gF, WM span, and lure accuracy. Color of regions reflects z-statistic for correlation between lure-related activity and WM span. Scatterplots demonstrate the correlations of the lure activity factor scores with gF, WM span, and lure accuracy respectively.
To determine the proportion of the gF variance that could be explained by the lure activity and WM span factors, we utilized the variance-partitioning method described in the Methods (see Figure 1c). As reported above, WM span captured 34.6% of the gF variance. The gF variance captured by lure activity was statistically significant, relating to 9.2% of the overall gF variance. The vast majority of the variance captured by lure activity was shared with WM span (8.7% of gF variance), with the unique contribution of lure activity capturing only 0.5% of gF variance. Altogether, the lure activity factor overlapped with 25.1% of the overall variance shared between gF and WM span.
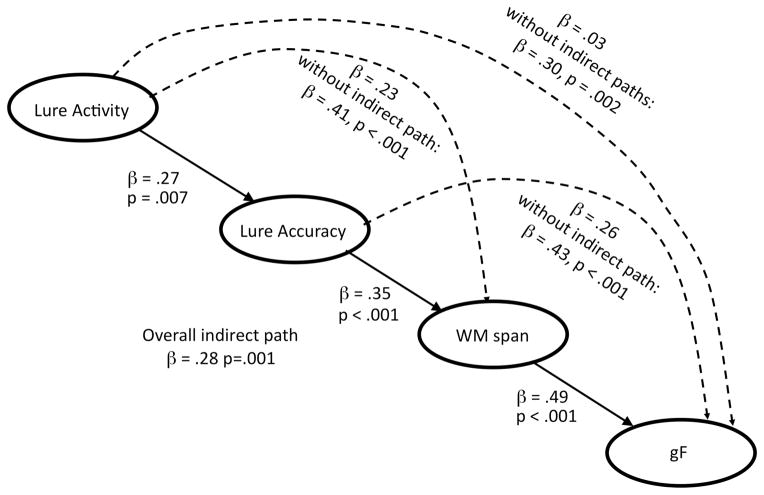
Path model analyses
For these analyses, we chose to adopt a reductionist framework in which the relationship between gF and WM span could be interpreted by their common relationships with simpler, mechanistically tractable factors. Therefore, we established a path model in which lure-related brain activity related to gF through its influences on lure accuracy and then WM span. We tested the significance of indirect paths from interference control brain activity through WM span (Figure 3) using bootstrap methods of Shrout & Bolger (2002) as implemented in Amos 7 (2000 iterations, bias-corrected 95% confidence interval). These analyses showed that the indirect paths from lure activity to gF through lure accuracy and WM span were statistically significant. The direct path from lure activity to gF was no longer significant after accounting for the indirect paths, and removing it from the model did not impair the model fit. Notably, these results were consistent with full mediation, in that the relationship of lure activity with gF could not be explained independently of its relationship with WM span. In addition, the indirect path from lure accuracy to gF through WM span was statistically significant. This indirect path through WM span partially mediated the lure accuracy – gF relationship, although the direct path from lure accuracy to gF remained significant. Altogether, these results suggest that a portion of the shared variance in gF and WM span may reflect neural mechanisms that mediate interference control ability, and their associated effects on behavioral performance.
Figure 3.
Path model predicting gF with indirect paths from lure activity and lure accuracy through WM span
Discussion
The current study found support for the hypothesis that interference control ability composes a portion of the relationship between gF and WM span. The results indicated that roughly 25% of the shared variance in gF and WM span was explained by individual differences in activity for high-interference lure trials within a set of brain regions including lateral PFC, medial PFC, and parietal lobe. Notably, this relationship with gF and WM span persisted after accounting for activity during lower-interference trials (i.e., targets and nonlures). Also, the relationship among interference control, gF, and WM span was not carried by sustained activation during the task blocks compared to rest. This pattern suggests that the activation during lure trials in these regions serves as a neural marker of the efficacy of interference control processes, rather than reflecting the larger construct of WM in general. Thus, these data suggest that interference control mechanisms may be a core component of variance measured by psychometric tests of gF and WM span.
The overall proportion of shared variance in the current study suggests that the constructs of interference control, WM span, and gF are not isomorphic. Nevertheless, the path analysis results suggest a plausible model in which interference control mechanisms account for individual variation in gF via a mediating influence on the construct of WM span. Specifically, the path analyses indicate that lure-related activity – within a core set of PFC, parietal, and other regions – predicts performance on these high-interference trials. This suggests that lure-related activity reflects the efficacy of interference control. In turn, interference control efficacy explains a significant portion of variance in WM span, which then explains a significant portion of variance in gF. As such, the path analysis significantly extends the work of Gray and colleagues (2003), which first put forth evidence that individual differences in gF can be at least partially explained in terms of interference control. The previous model is extended by placing WM span as an intermediary construct, operating between interference control and gF. Specifically, the work supports the further goal of mechanistic reductionism by indicating that the interference control construct may be more specific and neurally tractable than WM span, providing a more discrete measure that may reflect one component of individual variation in gF. Below we discuss further implications of the current results for research on interference control, WM span and gF.
The relationship between interference control, WM span, and gF
The current study builds on theoretical ideas originally put forth by Kane, Engle and colleagues (Kane & Engle, 2002). These researchers suggested that individual differences in gF and WM span might reflect variation in a common psychological process that they termed executive attention – the ability to maintain task representations in a highly accessible state in the face of interference. Further, they also suggested that neural mechanisms within DLPFC (BAs 9 and 46 within MFG) are essential for exerting executive attention, such that those mechanisms underlie the observed relationship between gF and WM span.
The current findings support and elaborate the executive attention account, while potentially clarifying the role of interference control. In particular, they directly support the idea that the management of interference is essential for the reliable maintenance and accessibility of information held in WM. Brain activity and performance on lure trials index how well interference is managed, capturing a critical component of what WM span measures. It is worth pointing out that our conclusions seem somewhat contradictory to previous work by Kane and colleagues (2007). Those authors found that n-back lure performance was poorly correlated with WM span (as measured by the OSPAN), and correlated more strongly with gF (as measured by the RAPM). Part of the apparent discrepancy may have arisen from numerous methodological differences between the two studies (e.g., type and proportion of lures, n-back stimulus types). Probably more important, however, is the fact that the current analysis strategy involved the extraction of latent factors from multiple WM span and gF tasks, rather than using single measures of those cognitive individual differences. This strategy likely increased the degree to which gF and WM span in the current study reflected variance in the core constructs as opposed to task-specific variance (Bollen, 1989). In agreement with Kane and colleagues (2007), we are not claiming that lure performance is an index of WM span, since lures capture only a portion of the WM span variance. Nonetheless, the results of the current study strengthen the conclusion that variability in interference control, as indexed by lure performance, captures a portion of variance that reflects a core component of the relationship between gF and WM span, thus suggesting a central role in cognitive individual differences.
The results of this study support the possibility that individual differences in gF and WM span are due to the efficacy of multiple different processes, rather than a single neural mechanism (i.e., interference control). Although the pattern of positive correlations (i.e., “positive manifold”) among cognitive tasks indicates a general intelligence factor, there have been suggestions that a general factor might not necessitate a single neural process underlying intelligence (e.g., Gould, 1981; Sternberg, 2000; Thomson, 1916). Four aspects of our data support this alternative view. First, it is important to emphasize that interference control did not capture all of the variance in gF or WM span, or even all of their shared variance. This fact demonstrates that understanding individual differences in these global constructs requires consideration of neurocognitive processes other than interference control. Second, the relationship of gF and WM span with brain activity in lateral PFC and parietal regions was specific to lure-related activity, and could not be explained by activity during target and nonlure trials in the n-back task. This finding accords with recent results demonstrating that blocked activation averaging across targets, lures, and nonlures failed to mediate the relationship between overall n-back performance and gF (Waiter et al., 2009). Altogether, this pattern suggests that the variance related to the recruitment of interference control processes is not part of a general cognitive factor that would affect performance on all trials in the n-back task. Third, our path analysis demonstrated that the influence of interference control on gF was fully mediated by indirect paths through WM span. These results suggest that neural mechanisms involved in interference control likely form the basis of some portion of the relationship between gF and WM span, but not the full relationship. Fourth, the brain regions implicated in the common variance in gF, WM span, and interference control did not capture all of the brain regions that have been implicated in gF, WM span, or interference control. For instance, anterior lateral PFC (BA10; also referred to as frontopolar cortex) has been implicated in the development of fluid reasoning (Ferrer, O’Hare, & Bunge, 2010) and in WM (Gilbert et al., 2006; Wager & Smith, 2003), but was not identified in the current study. Given that the vast majority of the overall variance in these cognitive individual differences was not captured by the commonalities with interference control ability, we believe that it will be beneficial for future research to adopt a similar framework to investigate other neurologically-tractable processes related to these cognitive individual differences factors.
Although our goal was to relate the broader constructs of gF and WM span to more tractable mechanisms involved in interference control, we cannot ascertain the extent to which the results of the current study generalize to other interference control paradigms. We chose to investigate interference control mechanisms within the context of the n-back WM task based on a prior study from our laboratory that demonstrated a relationship with gF (Gray, Chabris, & Braver, 2003). However, there are numerous other interference control tasks that have been utilized in the behavioral and neuroimaging literature (c.f., Nee, Wager, & Jonides, 2007), and which would have made potential candidates for investigation within this experimental context.
The strongest experimental design would be one that parallels the approach we employed with regard to behavioral measures of individual difference constructs, by monitoring brain activity during multiple interference control tasks. Such an approach might enable the derivation of a “latent variable” reflecting interference control with more desirable psychometric properties (Bollen, 1989). We chose not to utilize this approach in the current study primarily for practical and logistical reasons, given the time constraints and increased complexity of the imaging environment. However, this would be an attractive target for future work in this area. The collection of neuroimaging data on multiple interference control tasks would permit an investigation of general versus specific interference control mechanisms related to gF and WM span.
It is worth noting that the reductionist framework we have adopted for investigating the nature of gF, WM span, and their common variance is only a useful starting point. Indeed, this approach tends to promote implicit claims about the causal nature of the relationships among these constructs that are not apparent from the data itself. While we do want to be clear that variation in cognitive ability related to gF and WM span appears to relate to the ability to exert control over interference, we do not intend to overstate the case by claiming that interference control mechanisms cause variation in WM span and gF. Since individual differences factors are qualities of the individual rather than variables that can be independently manipulated, it will be very difficult to definitively demonstrate the basis of these relationships without falling victim to errors of logic such as the “causal arrow” or “third variable” problems.
The nature of interference control mechanisms
Although our results clearly link interference control processes with gF and WM span, it is not entirely clear how activity within these PFC and parietal regions results in interference control. Recently, we have suggested the possibility of dual mechanisms of cognitive control: proactive control mechanisms engaged in advance to prevent interference, and reactive control mechanisms engaged in response to resolve interference (Braver et al., 2007). Consistent with those possibilities, there are a number of ways in which the neural mechanisms within PFC and parietal regions may promote control over interference. Interference control mechanisms could activate in advance of interference – namely, proactive control – to prevent inappropriate representations from entering WM and affecting performance. Alternatively, interference control mechanisms could be activated in response to the onset of interference in WM – namely, reactive control – to squelch interfering representations and/or amplify task-appropriate processes, thereby protecting performance.
In a recent study (Burgess & Braver, 2010), focusing on the temporal dynamics of interference control using the recent negatives task (Burgess & Braver, 2010), we found that the neural mechanisms of interference control were flexible and dependent on the degree to which interference could be predicted prior to its occurrence (i.e., interference expectancy). Specifically, the neural signature of reactive control – transient activation specifically in response to recent negatives – was most prominent under conditions in which the expectation of interference was low. In contrast, when interference expectancy was high, a more proactive neural signature, in which activity was increased following encoding and prior to probe onset, was observed. Most interestingly, high gF individuals showed a greater tendency to exhibit the proactive control pattern, particularly in the high expectancy condition.
Due to the continuous nature of the n-back task, it is difficult to determine conclusively whether interference control is exerted proactively or reactively in the current study. Because the presence of interference (i.e., lure trials) is random and unpredictable, one might expect proactive control to be engaged across all trials, either in a sustained fashion or transiently on all trials, in order to be prepared for interference. Consequently, the observed pattern of correlations specific to lure trials seems to suggest that gF and WM span relate to reactive control over interference. However, on lure trials, there are also simultaneous, proactive control processes necessary to update WM representations in preparation for future trials. Activity on lure trials may be affected by the ability to engage or maintain these proactive processes in the face of interference. Therefore, it is plausible that the relationship among gF, WM span, and interference control reflects proactive control over WM updating in the face of interference. Future research will be necessary to better establish whether the relationship between gF and WM span is related to proactive control, reactive control, or perhaps to the ability to dynamically switch between these modes.
In addition to our prior work suggesting that the temporal dynamics of activation is a critical dimension of interference control, further understanding is required regarding how control over interference is actually achieved in the brain. Computational models suggest that an emergent property of activating abstract task-set or goal representations is the promotion of task-relevant processing, and consequent reduction in the tendency for task-irrelevant information to interfere with that processing. These models, which are variously referred to as biased competition (cf., Cohen, Dunbar & McClelland, 1990; Desimone & Duncan, 1995), guided activation (Miller & Cohen, 2001), or goal maintenance (Braver, Barch & Cohen, 2002) models, have provided a useful account of how the same PFC mechanisms might enable interference control (Cohen et al., 1990), learn abstract task rules (Rougier, Noelle, Braver, Cohen, & O’Reilly, 2005), and actively maintenance information in WM (Braver, Barch & Cohen, 2002; Hazy, Frank, & O’Reilly, 2006). With respect to the current study, one hypothesis that remains to be tested is that individual differences in interference control, WM span, and gF may reflect the efficacy with which biased competition is implemented during the n-back task.
From the perspective of biased competition models, more robust activation of task-set or goal representations could result in better performance. In contrast, some studies have demonstrated relationships between activity and performance indicating that individual differences in cognitive ability reflect more efficient processing (i.e., reduced activation in higher performing individuals; Haier et al., 1988; Rypma & Prabhakaran, 2009). One explanation of this potential discrepancy is that individuals with higher cognitive ability possess more direct and specific interregional connectivity, possibly due to structural changes in white matter tracts (Rypma & Prabhakaran, 2009). Under this account, it would take less activation in PFC regions to produce the same influence (or bias) on task-relevant processing regions. An alternative possibility again relates to the temporal dynamics of PFC activity. In particular, if reduced task-set activation permits both task-relevant and task-irrelevant processing, then increased task-set activation within PFC prior to trial onset (i.e., proactive control) might be associated with faster performance and overall reduced activity. In contrast, if task-set activation is not engaged in PFC until after trial-onset (i.e., reactive control), one might expect poorer performance (slower RT) and greater overall activation in order to resolve conflict between task-relevant and task-irrelevant processing. These relationships between trial-by-trial RT and activity at different time periods within a trial have been demonstrated in several studies (Braver, Reynolds, & Donaldson, 2003; Weissman, Roberts, Visscher, & Woldorff, 2006, Yarkoni et al., 2009). Therefore, it is still unclear whether better cognitive ability must be the result of structural differences that give rise to greater neural efficiency. Instead, it is possible that more efficient task-related processing could result from more efficient temporal allocation of cognitive control, perhaps independently of and in addition to any structural differences associated with higher cognitive ability.
The specific portions of lateral PFC involved in representing task sets may depend upon the degree to which task performance requires representations that are abstract versus concrete (Badre & D’Esposito, 2007; Koechlin & Summerfield, 2007; O’Reilly, 2010). Results from a previous study (Badre & D’Esposito, 2007) suggest that lateral PFC may be organized along a rostral-caudal axis according to the level of abstraction required for a task set to guide action. The results of their study indicate that dorsal premotor cortex (PMd; posterior to dorsolateral PFC within precentral gyrus, BA 6) is involved in selecting a specific response based on the stimulus-response mapping (SR mapping) and that anterior dorsal premotor cortex (pre-PMd; a posterior portion of what we have termed dorsolateral PFC, within posterior MFG, BA 6 and 9) was involved in selecting between possible SR mappings depending upon some feature of the stimulus. The regions observed in the current results within bilateral dorsolateral PFC (MFG, BA 6 and 9) fall near the pre-PMd regions in the Badre & D’Esposito (2007) study (those authors reserve the term dorsolateral PFC for more anterior regions in BA 46). The activation of bilateral dorsolateral PFC in the current study may support interference control by selecting between a SR mapping based on temporal order (e.g., 3-back) and a SR mapping based on familiarity of the stimulus (e.g., seen recently). If only target and nonlure trials were present in the experiment, either set of SR mappings would lead to the correct response. However, for lure trials, one must select their response based on the temporal order and not on familiarity in order to be correct. Thus, there may be more activation of these bilateral dorsolateral PFC regions on lure trials because they are needed to resolve the competition between the two SR mappings activated during those trials. Individuals with high gF/WM may activate these regions more robustly, and as such resolve the competition more effectively.
An intriguing hypothesis is that the anatomical location of lateral PFC regions related to gF, WM span, and interference control may depend upon the level of abstraction at which this interference occurs. Although they were not identified in the current study, the literature has implicated more anterior regions of lateral PFC – such as anterior dorsolateral PFC (BA 46) and anterior PFC (BA 10, also called frontopolar cortex) – in gF and WM, and to a lesser extent to interference control. If, for some tasks, interference control requires the use of more abstract representations (e.g., dimensional or contextual representations in the terminology of Badre & D’Esposito), then one might predict not only more robust activation in corresponding lateral PFC regions (anterior dorsolateral PFC and anterior PFC/frontopolar cortex), but also that the activation of these regions could covary with both cognitive individual differences and measures of interference control. Alternatively, even though tasks might involve more abstract, higher-order forms of interference control, the relationship between gF, WM span, and interference control may still be mediated by the more posterior dorsolateral PFC regions identified in the current study. Stated differently, posterior dorsolateral PFC may be a bottleneck for the implementation of interference control, “downstream” from more abstract task-set representations, which results in a fundamental constraint in all three constructs. Future studies may be able to address whether the level of task-set abstraction moderates the anatomical location of PFC regions underlying the relationship of gF and WM span with interference control.
Component processes within WM span measures
Complex WM span tasks require simultaneous processing and storage of items, but these abilities are not measured independently in traditional WM span measures. Consequently, it is not clear from the current study the degree to which interference control relates independently to processing versus storage capacity. A sizeable number of studies (e.g., Caplan & Waters, 1999; Daneman & Hannon, 2007; Duff & Logie, 2001; Logie & Duff, 2007; Waters & Caplan, 1996; 2003) have demonstrated that when separate behavioral measures of processing and storage capabilities are acquired during complex WM span tasks, those measures are actually poorly associated, and that it is the processing measures that capture additional variance in measures of general cognitive ability. Our perspective on the nature of interference control mechanisms suggests that they may be capable of affecting both processing and storage ability, as suggested by biased competition models (e.g., Miller & Cohen, 2001). However, this hypothesis will need to be specifically tested in future research.
In addition, our behavioral analyses suggest that measures of spatial WM span may be related more strongly to common, canonical measures of gF than are measures of verbal WM span. However, the reason for the stronger relationship is unclear. It is possible that the additional shared variance is simply related to task modality, in that both gF and spatial WM span are measured using spatial stimuli. On the other hand, it is also possible that gF and spatial WM span tap central executive processes to a greater extent than verbal WM span. Furthermore, the interference control mechanisms within spatial WM have not been investigated to the same extent as interference control within verbal WM. Future studies should investigate whether the relationships between gF, WM span, and interference control are domain-general, or if they differ within verbal and spatial modalities.
Training gF and WM span
The contribution of studies such as ours is not only to increase theoretical understanding of the mechanisms and constructs that contribute to global cognitive individual differences such as gF and WM span, but also to advance the applied goal of developing potential methods for enhancing cognitive abilities and performance. The two goals are clearly synergistic; a better understanding of the core psychological and neural mechanisms underlying cognitive individual differences factors may provide a more effective target for training interventions, and for predicting the long-term success and transfer of such interventions. Previous attempts to improve general cognitive abilities in healthy adults have resulted in limited success. Typically, the benefits that result from training on specific tasks fail to transfer to performance on other cognitive tasks. Despite the fact that gF predicts performance across cognitive domains, training on gF tasks does not transfer to novel tasks (Ackerman, 1987). Interestingly, recent attempts to train WM processes have resulted in transfer of benefits to gF measures (Jaeggi, Buschkuehl, Jonides, & Perrig, 2008; Westerberg & Klingberg, 2007). These findings suggest that it may be possible to improve general cognitive performance across various domains by training WM processes. Currently, however, there is insufficient understanding of the nature of changes to WM-related processes that transfer to gF, and which of those improved processes influences performance in demanding real-world tasks.
If interference control mechanisms form a key component of the gF - WM span relationship, then targeted training of interference control processes may lead to increased transfer to both WM span and gF. Initial findings from studies that have employed training interventions focusing on high-interference versions of WM tasks suggest that the training benefits transfer to other WM tasks (Persson & Reuter-Lorenz, 2008). However, it is not yet clear if such training benefits would also transfer to gF tasks, and more importantly, to executive function outside of the laboratory. The results from our current study would suggest that the degree of cognitive enhancement and transfer might depend upon the level of demands placed on interference control within in the training regimen. This idea is one that seems worthy of further investigation. Furthermore, our findings also suggest the degree of activity within brain regions involved in interference control, particularly lateral PFC and parietal cortex, may serve as a proximal marker of the efficacy of training techniques to improve general cognitive ability. Research to define additional processes relating gF and WM span to neurologically tractable constructs may ultimately identify further targets for training general cognitive ability, increasing the efficacy of cognitive training paradigms and their transfer to real-word domains.
Conclusions
In the last two decades, much progress has been made in cognitive and psychometric research towards understanding the relationships among important individual difference constructs such as gF and WM span, as well as their underlying mechanisms. The current study adds to this work, by demonstrating how such cognitive and psychometric investigations can be extended by including information from the neural level of analysis. Our findings suggest that the relationship between WM span and gF can be linked, at least in part, to a common dependence on mechanisms of interference control that reflect activation in a core set of brain regions centered on the lateral PFC and parietal cortex. The use of a large sample of subjects, as well as rigorous statistical and regression techniques, enabled us to demonstrate that these regions fairly specific and selective in their predictive utility for explaining common variance in gF and WM span. Nevertheless, it is also clear that, these interference control mechanisms do not fully explain WM span, gF or the relationship between the two constructs, suggesting that there may be multiple processes underlying these important dimensions of cognitive individual difference. We theorize that the exact brain regions and processes related to gF and WM span will likely depend upon the nature of processing required during the task, and the temporal dynamics of those task demands. This possibility would have implications not only for understanding individual differences in cognitive ability, but also for optimizing training paradigms to maximize their effectiveness and their transfer to real-world domains.
Acknowledgments
We thank Liz Chrastil and Christine Hoyer for their invaluable help with data collection, the CCP lab for helpful comments, and the participants for their time and effort. We also thank Robert Logie, Karen Mitchell, and three anonymous reviewers for their comments on a previous version of this article. This research was supported by National Institutes of Health grants R01 MH66088 to JRG and RO1 MH66078 to TSB.
References
- Ackerman PL. Individual differences in skill learning: an integration of psychometric and information processing perspectives. Psychological Bulletin. 1987;102:3–27. [Google Scholar]
- Ackerman PL, Beier ME, Boyle MO. Individual differences in working memory within a nomological network of cognitive and perceptual speed abilities. Journal of Experimental Psychology: General. 2002;131:567–589. [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cerebral Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. The role of prefrontal cortex in normal and disordered cognitive control: A cognitive neuroscience perspective. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford: Oxford University Press; 2002. pp. 428–447. [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–26. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Klingberg T, Jacobsen RB, Gabrieli JD. A resource model of the neural basis of executive working memory. Proceedings of the National Academy of Sciences. 2000;97:3573–3578. doi: 10.1073/pnas.050583797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Bunting M. The role of processing difficulty in the predictive utility of working memory span. Psychonomic Bulletin & Review. 2006;13:998–1004. doi: 10.3758/bf03213915. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Braver TS. Neural mechanisms of interference control in working memory: Effects of interference expectancy and fluid intelligence. PLoS ONE. 2010;5(9):e12861. doi: 10.1371/journal.pone.0012861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschkuehl M, Jaeggi SM. Improving intelligence: a literature review. Swiss Medical Weekly. 2010;140:19–20. 266–272. doi: 10.4414/smw.2010.12852. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters G. Working memory and sentence comprehension. Behavioral and Brain Sciences. 1999;22:77–126. doi: 10.1017/s0140525x99001788. [DOI] [PubMed] [Google Scholar]
- Cattell RB. Abilities: Their structure, growth, and action. Boston: Houghton Mifflin; 1971. [Google Scholar]
- Cattell RB. Measuring intelligence with the culture fair tests. Champaign, IL: The Institute for Personality and Ability Testing; 1973. [Google Scholar]
- Chein JM, Moore AB, Conway ARA. Domain-general mechanisms of complex working memory span. Neuroimage. 2011;54:550–559. doi: 10.1016/j.neuroimage.2010.07.067. [DOI] [PubMed] [Google Scholar]
- Chuah YM, Maybery MT. Verbal and spatial short-term memory: common sources of developmental change? Journal of Experimental Child Psychology. 1999;73:7–44. doi: 10.1006/jecp.1999.2493. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: an interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Colom R, Flores-Mendoza C, Rebollo I. Working memory and intelligence. Personality & Individual Differences. 2003;34:33–39. [Google Scholar]
- Colom R, Jung RE, Haier RJ. Finding the g-factor in brain structure using the method of correlated vectors. Intelligence. 2006;34:561–570. [Google Scholar]
- Colom R, Jung RE, Haier RJ. General intelligence and memory span: evidence for a common neuroanatomic framework. Cognitive Neuropsychology. 2007;24:867–878. doi: 10.1080/02643290701781557. [DOI] [PubMed] [Google Scholar]
- Colom R, Rebollo I, Palacios A, Juan-Espinosa M, Kyllonen PC. Working memory is (almost) perfectly predicted by g. Intelligence. 2004;32:277–296. [Google Scholar]
- Conway ARA, Cowan N, Bunting MF, Therriault DJ, Minkoff SRB. A latent variable analysis of working memory capacity, short term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30:163–183. [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Daneman M, Hannon B. What do working memory span tasks really measure? In: Osaka N, Logie RH, D’Esposito M, editors. The Cognitive Neuroscience of Working Memory. Oxford: Oxford University Press; 2007. pp. 21–42. [Google Scholar]
- Dempster FN, Corkill AJ. Individual differences in susceptibility to interference and general cognitive ability. Acta Psychologica. 1999;101:395–416. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proceedings of the National Academy of Sciences. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Shamosh NA, Green AE, Braver TS, Gray JR. Intellect as distinct from Openness: Differences revealed by fMRI of working memory. Journal of Personality and Social Psychology. 2009;97:883–892. doi: 10.1037/a0016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience: Brain structure and the Big Five. Psychological Science. 2010;21:820–828. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Duff SC, Logie RH. Processing and storage in working memory span. Quarterly Journal of Experimental Psychology. 2001;54A:31–48. doi: 10.1080/02724980042000011. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Edin F, Klingberg T, Johansson P, McNab F, Tegner J, Compte A. Mechanism for top-down control of working memory capacity. Proceedings of the National Academy of Sciences. 2009;106:6802–6807. doi: 10.1073/pnas.0901894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, Braver TS. Anxiety and cognitive efficiency: Differential modulation of transient and sustained neural activity during a working memory task. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:239–253. doi: 10.3758/CABN.8.3.239. [DOI] [PubMed] [Google Scholar]
- Ferrer E, O’Hare ED, Bunge SA. Fluid reasoning and the developing brain. Frontiers in Neuroscience. 2010;3:46–51. doi: 10.3389/neuro.01.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Geake JG, Hansen PC. Neural correlates of intelligence as revealed by fMRI of fluid analogies. NeuroImage. 2005;26:555–564. doi: 10.1016/j.neuroimage.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS. Why g matters: The complexity of everyday life. Intelligence. 1997;24:79–132. [Google Scholar]
- Gould SJ. The mismeasure of man. Harmondsworth, UK: Penguin; 1981. [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Tang C, Abel L, Buchsbaum MS. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence. 1992;16:415–426. [Google Scholar]
- Haier RJ, Siegel BV, Nuechterlein KH, Hazlett E, Wu JC, Paek J, et al. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–217. [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Martin RC. Dissociations among tasks involving inhibition: a single-case study. Cognitive, Affective & Behavioral Neuroscience. 2005;5:1–13. doi: 10.3758/cabn.5.1.1. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Banishing the homunculus: making working memory work. Neuroscience. 2006;139:105–118. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Science. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Marshuetz C, Smith EE, Reuter-Lorenz PA, Koeppe RA, Hartley A. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. Journal of Cognitive Neuroscience. 2000;12:188–196. doi: 10.1162/089892900561823. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Science. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Miura TK, Colflesh GJ. Working memory, attention control, and the N-back task: A question of construct validity. Journal of Experimental Psychology: Learning Memory and Cognition. 2007;33:615–622. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: a latent-variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology: General. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and non-verbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends in Cognitive Sciences. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kondo H, Morishita M, Osaka N, Osaka M. Functional roles of the cingulo-frontal network in performance on working memory. Neuroimage. 2004;21:2–14. doi: 10.1016/j.neuroimage.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Kucera, Francis WN. Computational Analysis of Present-Day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Kyllonen PC, Christal RE. Reasoning ability is (little more than) working memory capacity?! Intelligence. 1990;14:389–433. [Google Scholar]
- Lattin J, Carroll JD, Green PE. Analyzing Multivariate Data. Pacific Grove, CA: Brooks-Cole; 2003. [Google Scholar]
- Lee KH, Choi YY, Gray JR, Cho SH, Chae JH, Lee S, et al. Neural correlates of superior intelligence: stronger recruitment of posterior parietal cortex. Neuroimage. 2006;29:578–586. doi: 10.1016/j.neuroimage.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Logie RH, Duff SC. Separating processing from storage in working memory operation span. In: Osaka N, Logie RH, D’Esposito M, editors. The cognitive neuroscience of working memory. Oxford, UK: Oxford University Press; 2007. pp. 119–135. [Google Scholar]
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. Journal of Experimental Psychology: General. 2001;130:199–207. doi: 10.1037//0096-3445.130.2.199. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Kane MJ. The role of interference in memory span. Memory & Cognition. 1999;27:759–767. doi: 10.3758/bf03198529. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Weber K, Gunter TC, Engle RW. Dissociable brain mechanisms for inhibitory control: Effects of interference content and working memory capacity. Cognitive Brain Research. 2003;18:26–38. doi: 10.1016/j.cogbrainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Minamoto T, Osaka M, Osaka N. Individual differences in working memory capacity and distractor processing: Possible contribution of top-down inhibitory control. Brain Research. 2010;1335:63–73. doi: 10.1016/j.brainres.2010.03.088. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Sylvester CY, Jonides J, Smith EE. Dissociable neural mechanisms underlying response-based and familiarity-based conflict in working memory. Proceedings of the National Academy of Science. 2003;100:11171–11175. doi: 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer AC, Fink A. Fluid intelligence and neural efficiency: Effects of task complexity and sex. Personality and Individual Differences. 2003;35:811–827. [Google Scholar]
- Neubauer AC, Fink A. Intelligence and neural efficiency: Measures of brain activation versus measures of functional connectivity in the brain. Intelligence. 2009;37:223–229. [Google Scholar]
- Neubauer AC, Fink A, Schrausser DG. Intelligence and neural efficiency: the influence of task content and sex on the brain–IQ relationship. Intelligence. 2002;30:515–536. doi: 10.1016/j.cogbrainres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Grabner RH, Freudenthaler HH, Beckmann JF, Guthke J. Intelligence and individual differences in becoming neurally efficient. Acta Psychologia. 2004;116:55–74. doi: 10.1016/j.actpsy.2003.11.005. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC. The What and How of prefrontal cortical organization. Trends in neurosciences. 2010;33:355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka M, Osaka N, Kondo H, Morishita M, Fukuyama H, Aso T, Shibasaki H. The neural basis of individual differences in working memory capacity: an fMRI study. Neuroimage. 2003;18:789–797. doi: 10.1016/s1053-8119(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Osaka N, Logie RH, D’Esposito M. The Cognitive Neuroscience of Working Memory. Oxford: Oxford University Press; 2007. [Google Scholar]
- Osaka N, Osaka M, Kondo H, Morishita M, Fukuyama H, Shibasaki H. The neural basis of executive function in working memory: an fMRI study based on individual differences. Neuroimage. 2004;21:623–631. doi: 10.1016/j.neuroimage.2003.09.069. [DOI] [PubMed] [Google Scholar]
- Osaka M, Komori M, Morishita M, Osaka N. Neural bases of focusing attention in working memory: an fMRI study based on group differences. Cognitive, Affective & Behavioral Neuroscience. 2007;7:130–139. doi: 10.3758/cabn.7.2.130. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Reuter-Lorenz PA. Gaining control: Training executive function and far transfer of the ability to resolve interference. Psychological Science. 2008;19:881–888. doi: 10.1111/j.1467-9280.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JA, Desmond JE, Glover GH, Gabrieli JD. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven’s Progressive Matrices Test. Cognitive Psychology. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Oxford, U.K: Oxford Psychologists Press; 1998. [Google Scholar]
- Rougier NP, Noelle D, Braver TS, Cohen JD, O’Reilly RC. Prefrontal cortex and the flexibility of cognitive control: Rules without symbols. Proceedings of the National Academy of Sciences. 2005;102:7338–7343. doi: 10.1073/pnas.0502455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The aging of working memory. Neuropsychology. 1994;8:535–543. [Google Scholar]
- Schmithorst VJ, Holland SK. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. NeuroImage. 2006;31:1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CD, Green AE, Reis DL, Conway ARA, Engle RW, Braver TS, Gray JR. Individual differences in delay discounting: Relation to intelligence, working memory, and frontopolar cortex. Psychological Science. 2008;19:904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shrout P, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: effects of performance and aging. Proceedings of the National Academy of Sciences. 2001;98:2095–2100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AZ. Difference image versus ratio image error function forms in PET-PET realignment. In: Bailer D, Jones T, editors. Quantification of Brain Function Using PET. San Diego: Academic Press; 1996. [Google Scholar]
- Sternberg RJ. Cognition. The holey grail of general intelligence. Science. 2000;289:399–401. doi: 10.1126/science.289.5478.399. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Suess HM, Oberauer K, Wittmann WW, Wilhelm O, Schulze R. Working-memory capacity explains reasoning ability--and a little bit more. Intelligence. 2002;30:261–288. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, et al. Effects of frontal lobe damage on interference effects in working memory. Cognitive, Affective & Behavioral Neuroscience. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Thomson GH. A hierarchy without a general factor. British Journal of Psychology. 1916;8:271–281. [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. NeuroImage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective & Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Deary IJ, Staff RT, Murray AD, Fox HC, Starr JM, Whalley LJ. Exploring possible neural mechanisms of intelligence differences using processing speed and working memory tasks: An fMRI study. Intelligence. 2009;37:199–206. [Google Scholar]
- Waters G, Caplan D. The measurement of verbal working memory capacity and its relation to reading comprhension. Quarterly Journal of Experimental Psychology. 1996;49A:51–79. doi: 10.1080/713755607. [DOI] [PubMed] [Google Scholar]
- Waters G, Caplan D. The reliability and stability of verbal working memory measures. Behavior Research Methods, Instruments and Computers. 2003;35:550–564. doi: 10.3758/bf03195534. [DOI] [PubMed] [Google Scholar]
- Welborn BL, Papademetris X, Reis DL, Rajeevan N, Bloise SM, Gray JR. Variation in orbitofrontal cortex volume: Relation to sex, emotion regulation, and affect. Social Cognitive & Affective Neuroscience. 2009 doi: 10.1093/scan/nsp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Klingberg T. Changes in cortical activity after training of working memory - a single-subject analysis. Physiology & Behavior. 2007;92:186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. general methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo T, Braver TS. BOLD correlates of trial-by-trial response time variability in gray and white matter: a multi-study fMRI analysis. PLoS ONE. 2009;4(1):e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]