Abstract
The lymphocytic choriomeningitis virus (LCMV) system constitutes one of the most widely used models for the study of infectious disease and the regulation of virus-specific T cell immunity. However, with respect to the activity of costimulatory and associated regulatory pathways, LCMV-specific T cell responses have long been regarded as relatively independent and thus distinct from the regulation of T cell immunity directed against many other viral pathogens. Here, we have reevaluated the contribution of CD28-CD80/86 costimulation in the LCMV system by use of CD80/86-deficient mice, and our results demonstrate that a disruption of CD28-CD80/86 signaling compromises the magnitude, phenotype, and/or functionality of LCMV-specific CD8+ and/or CD4+ T cell populations in all stages of the T cell response. Notably, a profound inhibition of secondary T cell immunity in LCMV-immune CD80/86-deficient mice emerged as a composite of both defective memory T cell development and a specific requirement for CD80 but not CD86 in the recall response, while a related experimental scenario of CD28-dependent yet CD80/86-independent secondary CD8+ T cell immunity suggests the existence of a CD28 ligand other than CD80/86. Furthermore, we provide evidence that regulatory T cells (TREGs), the homeostasis of which is altered in CD80/86−/− mice, contribute to restrained LCMV-specific CD8+ T cell responses in the presence of CD80/86. Our observations can therefore provide a more coherent perspective on CD28-CD80/86 costimulation in antiviral T cell immunity that positions the LCMV system within a shared context of multiple defects that virus-specific T cells acquire in the absence of CD28-CD80/86 costimulation.
INTRODUCTION
The generation of specific T cell immunity is governed by multiple determinants that shape the proliferative expansion and functional maturation of effector T cells (TE) as well as their subsequent differentiation into memory T cells (TM). Conceptualization of these processes permits the straightforward demarcation of T cell receptor (TCR)-peptide/major histocompatibility complex (MHC) interactions (“signal 1”), yet the simple notion of a defined “costimulus” required for the optimization of specific T cell responses, historically referred to as “signal 2,” has been eroded by the realization that a multiplicity of diverse receptor-ligand interactions between T cells and antigen-presenting cells (APCs), soluble factors (e.g., cytokines), and specific temporospatial constraints operate in concert to control the eventual magnitude as well as the molecular, phenotypic, and functional properties of responding TE populations. Thus, it is the integration of signals derived from a large complex of stimulatory and inhibitory interactions that permits activated T cells the translation of minimal kinetic alterations into profound modifications of the ensuing T cell response (26, 84). Insofar as these interactions produce a kinetic, quantitative, and/or qualitative enhancement of specific T cell immunity, individual components within this complex may be referred to as costimulatory. Nevertheless, such conclusions, as illustrated by the at times confusing and seemingly contradictory observations reported throughout the history of costimulation research, have to be tempered by the inevitable limitations of the particular experimental methodologies and model systems that may or may not reveal evidence for relevant costimulatory interactions in the generation of specific T cell immunity and associated clinical symptomatology.
A case in point is the costimulatory triad of CD28, CD80 (B7.1), and CD86 (B7.2), the role of which has been explored in numerous experimental settings. In fact, as judged by the sheer number of relevant publications within the past 2 decades, this triad, together with the inhibitory CD80/86 receptor cytotoxic-T lymphocyte (CTL)-associated antigen 4 (CTLA-4), arguably constitutes the best-characterized receptor-ligand system in the realm of costimulation, yet even in the more restricted context of CD28-CD80/86 costimulation and its impact on the regulation of antiviral T cell immunity (6, 80), the proposal of certain ground rules, while certainly sensible at the time of their formulation as based on the available scientific evidence, has subsequently encountered numerous exceptions such that their continued utility has to be reevaluated. To date, infections with multiple distinct and related viruses, escalating dosages, and various challenge routes have been employed to ascertain the role of CD28-CD80/86 costimulation preferentially in CD28−/− mice but also complemented by analyses of CD80−/− and/or CD86−/− strains as well as ligand (anti-CD80/86 and CTLA-4Ig) and receptor (anti-CD28 and anti-CTLA-4) blockade. These viruses include LCMV (1, 15, 25, 28, 36, 44, 45, 65, 69, 72, 73, 81); vesicular stomatitis virus (VSV) (1, 15, 43–45, 58, 69); vaccinia virus (VACV) (21, 23, 24, 45, 67, 70) and the related ectromelia virus (ECTV) (21); influenza A virus (5, 7, 8, 10, 14, 29, 32, 51, 53, 74), herpes simplex viruses (herpes simplex virus 1 [HSV-1] and HSV-2) (10, 20, 75, 76); murine gammaherpesvirus 68 (MHV-68) (18, 22, 24, 42, 47, 54); polyomavirus (PyV) (41), murine cytomegalovirus (MCMV) (2, 3, 17); and adenovirus (28), and part of this work has been instrumental in establishing three prominent paradigms: (i) the requirement for costimulation to generate effective primary TE immunity is inversely correlated to the TCR stimulus strength and duration (45, 69), (ii) CD4+ TE responses are more reliant on costimulation than are CD8+ TE responses (80), and (iii) secondary, in contrast to primary, TE immunity is relatively costimulation independent (71). Notable deviations from these rules, however, have been reported over the past decade.
Regarding the first paradigm, the notion that CD28-CD80/86 costimulation is required for the effective generation of primary CD8+ TE responses directed against abortively replicating viruses (VSV, attenuated VACV, and influenza A virus) but not more widely replicating viruses (LCMV and wild-type [wt] VACV) (5, 7, 8, 10, 14, 29, 32, 45, 51, 53, 69) needs to be reconciled with more recent evidence that the regulation of T cell immunity under conditions of extensive viral replication, persistence, and/or latency does not necessarily refrain from the productive engagement of CD28-CD80/86 costimulation. For example, work with different LCMV strains and experimental protocols (15, 72, 81), MHV-68, PyV, and MCMV (3, 22, 41) documented that interference with the CD28-CD80/86 pathway can produce a variety of defects that range from subtle alterations of specific CD8+ TE responses to an outright failure of virus control. Perhaps most impressively, CD28−/− mice were highly susceptible to lethal mousepox as a consequence of delayed and reduced CD8+ TE responses in the wake of infection with the natural mouse pathogen ECTV (21). In regard to the second paradigm, although considerably less attention has been devoted to the role of CD28-CD80/86 signaling in the generation of antiviral CD4+ TE immunity, the reduction of primary CD4+ TE responses specific for LCMV (15, 28, 44, 65, 73), influenza virus (8), VSV (44), HSV-1 (20), and MCMV (2) observed for CD28−/− or CD80/86−/− mice or under conditions of CD80/86 blockade supports the concept that the effective generation of CD4+ T cell immunity is critically dependent on CD28-CD80/86 costimulation. Interestingly, though, VACV-specific CD4+ TE responses elicited in CD80/86−/− mice were reported to be normal, but the possible reasons for this particular divergence from all other viral model systems remain rather speculative (23). For the third paradigm, as reviewed previously by Boesteanu and Katsikis (9), the development of the concept that secondary TE responses are less dependent on CD28-CD80/86 costimulation than primary TE responses was based in large part on in vitro experiments. Nevertheless, some early observations suggested that secondary virus-specific CD8+ TE immunity was adversely affected by impaired CD28-CD80/86 costimulation, as shown by the reduced secondary CTL activity of VSV-specific CD8+ TM generated in CD80/86−/− mice (58) or of influenza virus- and LCMV-specific CD8+ TM originally primed under conditions of CD80/86 blockade (36, 51). Similarly, MHV-68-immune CD28−/− and CD80/86−/− mice shared certain phenotypic and functional CD8+ TM alterations that were associated with reduced secondary expansions following a recombinant VACV (rVACV) challenge (22). In more recent work, potential problems arising from the generation of virus-specific CD8+ TM populations in immunodeficient mice were circumvented by the adoptive transfer (AT) of wt CD8+ TM into CD80/86−/− hosts and/or the administration of a costimulation blockade (anti-CD28 or CTLA-4Ig) specifically in the context of secondary challenges. Collectively, those studies demonstrated that influenza virus-, HSV-1-, VACV-, LCMV-, and MHV-68-specific CD8+ TM primed in wt mice require signaling via the CD28-CD80/86 axis for efficient secondary expansion (10, 24, 25, 28) and virus clearance (10, 25). In addition, the generation of VACV-, MHV-68-, or LCMV-specific CD8+ TM in CD28−/− or CD80/86−/− mice compromised their secondary reactivity after AT into wt hosts (24, 28), indicating the acquisition of certain CD8+ TM-intrinsic functional defects that, in the case of CD28−/− VACV-specific CD8+ TM, could be reversed only by interleukin-2 (IL-2) immune complex treatment during the recall phase (24). Since similar costimulation requirements also seem to apply to influenza virus-, VACV-, and LCMV-specific CD4+ TM (23, 25, 74), it would appear that the optimal elaboration of secondary antiviral T cell immunity at large is indeed dependent on productive CD28-CD80/86 interactions.
Adding to the interpretive challenges of the above-described observations are certain discordances reported for the degree of impaired T cell immunity, associated virus control, and clinical symptomatology in CD28- versus CD80/86-deficient or -blocked mice. For example, paralysis following local HSV-1 infection was aggravated by CTLA-4Ig-mediated CD80/86 blockade but not in CD28−/− mice (20). Similarly, viral reactivation occurred in the lungs of MHV-68-infected CD80/86−/− but not CD28−/− (22, 42, 54), anti-CTLA-4-treated CD28−/− (22), or CD28−/−/CTLA-4−/− mice (18), leading to the postulate of an unidentified (activating) receptor for CD80/86, a notion that has also been advanced in models of cardiac allograft transplantation (55, 82). Most recently, primary MCMV-specific CD8+ TE responses were shown to be somewhat more compromised in CD80/86−/− or anti-CD80/86 antibody-treated wt mice than in CD28−/− mice (3), and exacerbated phenotypic alterations and functional defects of CD8+ TM populations combined with impaired virus control in CD80/86−/− but not CD28−/− mice infected with the more virulent LCMV Traub strain have prompted similar speculations about additional CD80/86 receptors (28). Another study, based on the finding that a CD80/86 blockade by CTLA-4Ig treatment substantially delayed LCMV Armstrong (Arm) clearance in CD28−/− mice, suggested the possibility of an activating rather than an inhibitory CTLA-4 function (65). Although a similar interpretation was proposed for some nonviral model systems (50), this observation is also compatible with the notion of an activating CD80/86 receptor other than CTLA-4 or CD28. A novel CD80 receptor has in fact been identified in both mice and humans (PD-L1/CD274/B7-H1), yet CD80–PD-L1 interactions appear to promote inhibitory rather than activating T cell signals (12, 13). Together with the recent identification of another costimulatory CD28 ligand in humans (ICOSL/B7-H2) and the description of the Trem-like transcript 2 protein (TLT2) as a potential CD276/B7-H3 receptor (31, 48, 83), these observations clearly point toward more “promiscuous” receptor-ligand interactions within and beyond the B7 family that may well contribute to the regulation of antiviral T cell immunity under certain experimental and naturally occurring conditions.
In light of these considerations, the consequential need to investigate T cell immunity to disparate viral pathogens in detail, and the important role of the LCMV system in both establishing the reigning CD28-CD80/86 paradigms as well as emphasizing some of the notable exceptions, it is perhaps somewhat surprising that but a single and very recent report has evaluated LCMV-specific T cell immunity directly in CD80/86−/− mice (28). We have therefore extended these investigations to further define the precise nature of defects imparted on specific CD8+ and CD4+ T cell populations by genetic CD80/86 deficiency. Our results demonstrate that the development of LCMV-specific T cell immunity, far from being independent of CD28-CD80/86 costimulatory and associated regulatory pathways, is indeed subject to CD28-CD80/86 control at all stages of the immune response and thus can assist in the conceptual consolidation of CD28-CD80/86 costimulation in antiviral T cell immunity as well as the delineation of clinically important parameters that may or may not be affected by a therapeutic costimulation blockade.
MATERIALS AND METHODS
Mice, viruses, and infections.
C57BL6 (B6), congenic B6.CD90.1 (B6.PL-Thy1a/CyJ), and B6.CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ) mice as well as CD80-deficient (B6.129S4-Cd80tm1Shr/J), CD86-deficient (B6.129S4-Cd86tm1Shr/J), CD80/86-deficient (B6.129S4-Cd80tm1Shr Cd86tm2Shr/J), and CD28-deficient (B6.129S2-Cd28tm1Mak/J) mice on the B6 background were purchased from the Jackson Laboratory. p14 T cell receptor transgenic (TCRtg) mice recognize the immunodominant LCMV-GP33-41 epitope restricted by Db (63) and were obtained on a B6.CD90.1 background from M. Oldstone (The Scripps Research Institute); OT-I TCRtg mice [C57BL/6-Tg(TcraTcrb)1100Mjb/J; Jackson Laboratory] recognize the ovalbumin257-264 determinant in the context of Kb and were bred with B6.CD90.1 mice to generate OT-I mice with a mixed congenic CD90.1/2 background. B6.DEREG (“depletion of regulatory T cell”) mice express a diphtheria toxin (DT) receptor-enhanced green fluorescent protein (GFP) fusion protein under the control of the foxp3 gene locus, permitting the targeted depletion of FoxP3+ regulatory T cells (TREGs) by DT treatment (46). LCMV Armstrong (Arm) clone 53b and clone 13 (cl13) were obtained from M. Oldstone, and stocks were prepared by a single passage on BHK-21 cells; plaque assays for the determination of virus titers were performed as described previously (40). For the quantification of LCMV-GP mRNA in mouse tissues, we employed a quantitative real-time PCR (qRT-PCR) assay, with slight modifications, as described previously by McCausland and Crotty (59); plasmid pSG5-GP used for the generation of standard curves was a gift from J.-C. de la Torre (The Scripps Research Institute). The qRT-PCR assay has an estimated ∼1,000-fold-increased sensitivity compared to standard plaque assays (59). Eight- to ten-week-old mice were infected with a single intraperitoneal (i.p.) dose of 2 × 105 PFU LCMV Arm, and LCMV Arm-immune mice were rechallenged with 2 × 106 PFU LCMV cl13 intravenously (i.v.); intracranial (i.c.) infections were performed with 103 PFU LCMV Arm. In some cases, naïve recipients of adoptively transferred LCMV-specific TM were challenged with 2 × 105 PFU Arm i.p. or 2 × 106 PFU cl13 i.v. All procedures were performed in accordance with NIH and University of Colorado Institutional Animal Care and Use Committee (IACUC) guidelines.
Lymphocyte isolation and purification, adoptive transfers (ATs), and stimulation cultures.
Lymphocytes were isolated from blood, spleen, and other tissues as described previously (38, 39). To generate p14 chimeras, CD8+ T cells from naïve p14 mice were enriched by negative selection using magnetic beads (purity, ∼95%) (EasySep; StemCell Technologies), and 2 × 103 p14 cells were transferred i.v. into sex-matched recipient mice that were challenged ∼24 h later with LCMV. All other ATs were performed with donor T cell populations enriched by B cell or combined CD4+ T/B cell depletion (anti-B220/CD4-phycoerythrin [PE] antibody followed by anti-PE magnetic beads; StemCell Technologies) and sex-matched recipients, as detailed in Fig. 4 and 6 to 8 and the corresponding figure legends. Short-term stimulation cultures (5 h) with LCMV-GP- and -NP-derived peptides (CD8+ T cell determinants, GP33, GP92, GP118, GP276, NP205, and NP396; CD4+ T cell determinants, GP64 and NP309) in the presence of the protein transport inhibitor brefeldin A (BFA) were performed as described previously (39) to evaluate inducible T cell functionalities. Please note that the CD4+ T cell population specific for the I-Ab-restricted GP64-80 determinant also reacts with longer (GP61-80) and shorter (GP66-77) versions of this epitope (37).
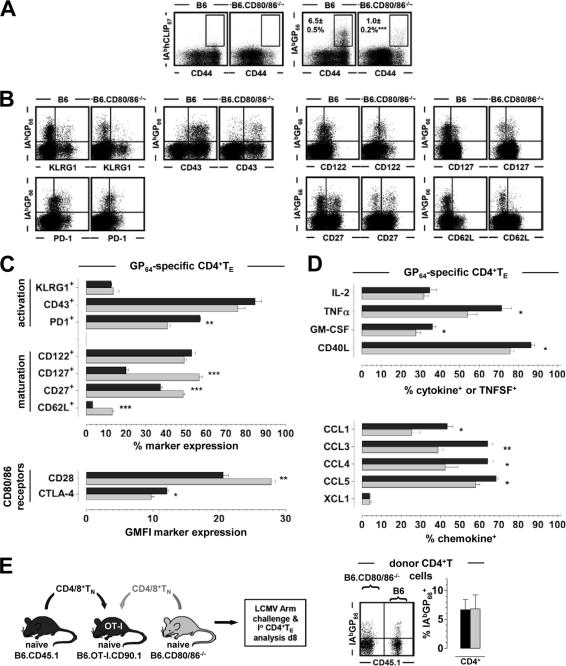
Fig 4.
Phenotypic and functional alterations of LCMV-specific CD4+ TE generated in the absence of CD80/86. (A) Eight days after LCMV challenge, spleen cells were stained with I-AbGP66 and control (I-AbhCLIP87) tetramers as detailed in Materials and Methods. Plots are gated on B220−/CD4+ T cells. Values indicate percentages (SEM) of I-AbGP66+ CD4+ TE; similar results were obtained with I-AbGP61-80 tetramers produced in the laboratory (not shown). (B and C) Phenotypes of GP66-specific CD4+ TE (day 8 after challenge). Dot plots are gated on B220−/CD4+ T cells. (D) Functional profiles of GP64-specific CD4+ TE evaluated after 5 h of in vitro peptide stimulation. The bar diagrams depict the fractions of GP64-specific (IFN-γ+) CD4+ TE synthesizing the indicated cytokines, TNFSFs, or chemokines. (E) Mixed peripheral chimeras generated by the combination of B cell-depleted B6.CD45.1 and B6.CD80/86−/− spleen cells at a ratio of 1:1 and AT into OT-I recipients heterozygous at the CD90 locus (CD90.1 × CD90.2). TCRtg OT-I mice, due to their restricted T cell repertoire, cannot generate LCMV-specific CD8+ or CD4+ TE responses such that all LCMV-specific T cells have to be recruited from the donor T cell pool (60; Eberlein and Homann, unpublished). Eight days after LCMV challenge, CD4+ donor TE expansions in peripheral blood were quantified (dot plot gated on CD90.1−/CD4+ donor CD4+ T cells); similar results were obtained for NP396-specific CD8+ TE, and the chimeric mice controlled the LCMV infection, as assessed by serum plaque assays (not shown). Representative data are SEM (for n ≥ 3 mice/group for all experiments).
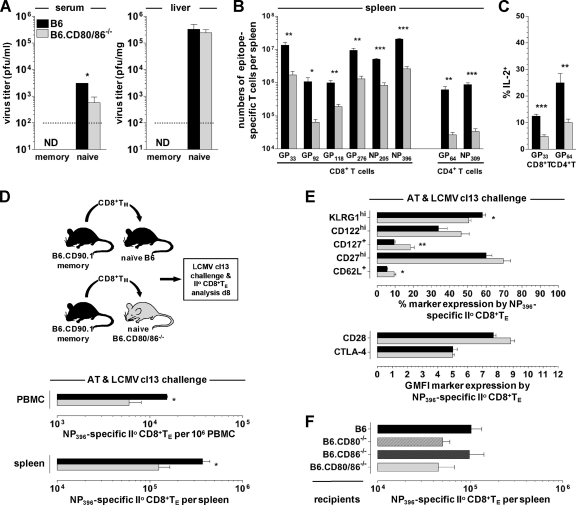
Fig 6.
CD80/86, immune protection, and secondary TE reactivity under conditions of LCMV cl13 rechallenge. (A) Naïve B6 and B6.CD80/86−/− as well as LCMV-immune (“memory”) B6 and B6.CD80/86−/− mice (93 dpi) were challenged with 2 × 106 PFU LCMV clone 13 i.v., and infectious virus titers in serum and liver were determined 6 days later. The dotted line indicates the detection threshold of 2 × 102 PFU/mg tissue. ND, not detected. Data are SEM for 3 to 4 mice/group. (B) Absolute numbers of secondary epitope-specific (IFN-γ+) CD8+ and CD4+ TE in the spleen cl13 rechallenge of LCMV Arm-immune mice (please note that these experiments were conducted 6 days after rechallenge, i.e., 2 days earlier than most of the subsequent analyses, which were performed on day 8). (C) Induced IL-2 production by secondary GP33-specific CD8+ and GP64-specific CD4+ TE. (D) CD8+ T cells were enriched from LCMV-immune wt donors (193 dpi) by combined CD4/B220 depletion, and populations containing 104 DbNP396+ CD8+ TM were transferred into naïve B6 or B6.CD80/86−/− recipients that were subsequently challenged with 2 × 106 PFU LCMV cl13 i.v. Secondary donor CD8+ TE expansions in blood and spleen were quantified 8 days later. (E) Phenotypes of secondary DbNP396+ CD8+ TE. (F) Experimental design as detailed above for panel D, using enriched LCMV-immune wt donor populations containing 2 × 103 DbNP396+ CD8+ TM (54 dpi) and the indicated naïve B6, B6.CD80−/−, B6.CD86−/−, and B6.CD80/86−/− recipients; cl13 rechallenge; and day 8 analysis (n = 3 mice/group for all AT experiments). II°, secondary.
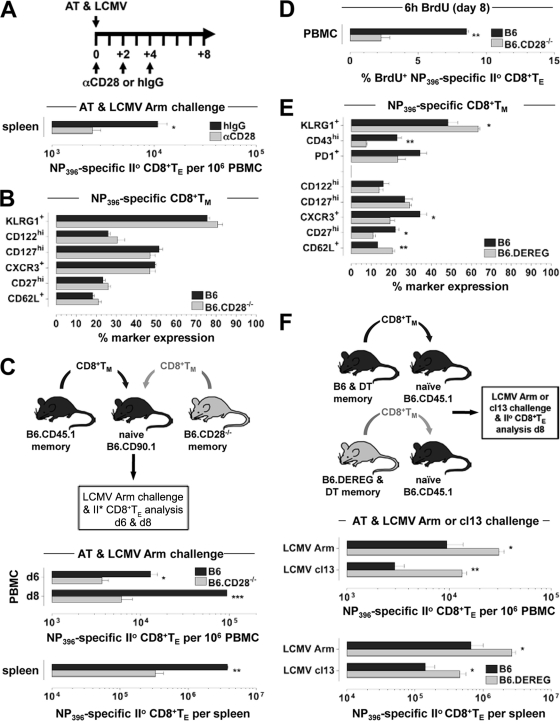
Fig 8.
Roles of CD28 and TREGs in regulation of secondary CD8+ TE proliferation and expansion. (A) AT/rechallenge experiments conducted with 2 × 103 DbNP396+ wt CD8+ TM (137 dpi) and congenic wt recipients in the presence of an anti-CD28 blockade or hamster IgG control treatment. The diagram depicts the schedule of antibody administration in relation to AT/LCMV Arm challenge on day 0 (analyses were performed on day 8). Similar results were obtained in 4 independent experiments (n = 3 mice/group for all AT experiments). (B) Phenotypes of specific CD8+ TM (53 dpi) in LCMV Arm-immune CD28-deficient mice. (C) CD8+ TM (∼340 dpi) were enriched from LCMV-immune B6.CD45.1 and B6.CD28−/− donors and mixed, and populations containing 104 DbNP396+ CD8+ TM each were transferred into naïve B6.CD90.1 congenic recipients prior to LCMV Arm challenge. Quantifications of secondary CD8+ TE expansions were conducted on day 6 and day 8. (D) Proliferation of secondary wt and CD28−/− CD8+ TE among peripheral blood mononuclear cells assessed following a 6-h in vivo BrdU pulse (8 dpi), as detailed in Materials and Methods. Similar results were obtained for CD8+ TE recovered from other tissues and/or specific for other LCMV determinants (not shown). (E) Phenotypes of blood-borne DbNP396+ CD8+ TM (59 dpi) in B6 and B6.DEREG mice treated with DT only during the early primary CD8+ TE response (day −1 to day +5) (Fig. 3F). (F) Splenic CD8+ T cells were enriched (CD4+ T/B cell depletion) from LCMV Arm-immune B6 versus B6.DEREG mice (66 dpi) originally treated with DT as indicated above, and CD8+ T cell populations containing 104 DbNP396+ CD8+ TM each were transferred into naïve CD45.1 congenic recipients that were subsequently challenged with LCMV Arm or cl13 and analyzed 8 days later. Note that the recipient mice maintained intact TREG compartments throughout these AT/rechallenge experiments (data are SEM for 3 mice/group). II°, secondary.
Antibodies, MHC tetramers, and flow cytometry.
All monoclonal antibodies were purchased as fluorophore-conjugated reagents from ebioscience, Biolegend, BDBiosciences, or RnD Systems; chemokine-specific polyclonal goat antibodies were obtained from RnD Systems. DbNP396-404, DbGP33-41, and I-AbGP66-77 MHC-peptide complexes were provided as biotinylated monomers and/or fluorophore-conjugated tetramers by the NIH tetramer core facility and used for the flow cytometry-based identification of LCMV-specific CD8+ and CD4+ T cells as described previously (39); essentially the same results were obtained by using I-AbGP66-77 and I-AbGP61-80 tetramers generated in the laboratory (not shown). The methodologies for cell surface (antibodies and MHC tetramers) and intracellular staining, including the detection of cytokines, members of the tumor necrosis factor superfamily (TNFSFs), CTLA-4, chemokines, and bromodeoxyuridine (BrdU), were detailed elsewhere previously (19, 35, 39, 49). For the identification of FoxP3+ cells, we used the reagents and protocols supplied with a FoxP3 staining kit (ebioscience). All samples were acquired on a FACSCalibur or LSR II flow cytometer (BDBiosciences) and analyzed with CellQuest, DIVA (BDBiosciences), and/or FlowJo (TreeStar) software.
In vivo treatment.
For selective TREG depletion, B6.DEREG (and B6 control) mice were injected i.p. with 1.0 μg DT (Sigma) on days −1, +2, and +5 relative to primary LCMV infection. The blockade of CD28 was performed by the i.p. injection of 100 μg anti-CD28 antibody (clone 37.51; BioXCell or Biolegend) or a hamster isotype control (Biolegend or Accurate Chemical) on days 0, +2, and +4 in relation to AT/LCMV rechallenge; the respective antibodies obtained from the different purveyors yielded equivalent experimental results (not shown). For the assessment of CD8+ TM homeostatic proliferation, LCMV-immune mice were injected with 2 mg BrdU (Sigma) i.p. and subsequently supplied with daily prepared drinking water containing 0.8 mg/ml BrdU for 7 days as described previously (49). The proliferation of secondary CD8+ TE was assessed after a 6-h BrdU pulse (2 mg BrdU i.p.) on day 6 or day 8 after CD8+ TM AT and rechallenge, and analysis of BrdU incorporation by specific T cells was performed as detailed elsewhere previously (49).
Statistical analyses.
Data handling, analysis, and graphic representation were performed by using Prism 4.0 software (GraphPad Software, San Diego, CA). All data summarized in bar diagrams are expressed as means ± 1 standard error (SE); asterisks indicate statistical differences calculated by unpaired Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
RESULTS
CD80 and CD86 expression by major immune cell subsets.
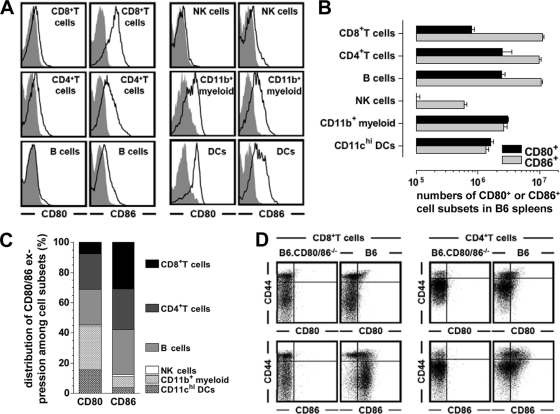
Although the major costimulatory molecules CD80 and CD86 are preferentially expressed by antigen-presenting cells (APCs), their expression by other cell types under various experimental conditions has long been appreciated (27). Here, using B6.CD80/86−/− mice as a negative control, we have reevaluated the expression patterns of CD80 and CD86 among major immune cell subsets obtained from unmanipulated B6 mice (Fig. 1A and B). Overall, it is noteworthy not only that CD80 and/or CD86 is expressed by subsets within every major immune cell compartment (T cells, B cells, NK cells, myeloid cells, and dendritic cells [DCs]) but also that T cells in particular constitute ∼30% of CD80- and ∼60% of all CD86-expressing immune cells under physiological steady-state conditions (Fig. 1C). In fact, the CD86+ phenotype appears to be a hallmark of resting naïve (CD44lo) CD8+ T cells in general (Fig. 1D). Thus, the rather “promiscuous” CD80/86 expression patterns have to be taken into account in studies that assess immune responses in the absence of systemic CD80/86 expression.
Fig 1.
CD80/86 expression by major immune cell subsets. Spleen cells from naïve B6 and B6.CD80/86−/− mice were stained for various surface markers as well as CD80 or CD86. (A) Histograms were gated on the indicated immune cell subsets by employing the following gating strategies: CD8+ T cells, CD3ϵ+ CD8+; CD4+ T cells, CD3ϵ+ CD4+; B cells, CD19+; NK cells, CD3ϵ− NK1.1+; CD11b+ myeloid cells, CD3ϵ− NK1.1− CD11b+ (containing monocytes, macrophages, neutrophils, and DC subsets); DCs, CD3ϵ−/CD11chi. Gray histograms, control strains (B6.CD80/86−/−); black tracings, experimental strains (B6). (B) Absolute numbers of CD80- or CD86-expressing cell subsets in the spleens of unmanipulated B6 mice. (C) Distribution of CD80 and CD86 expressions among immune cell subsets in B6 mice. The absolute numbers of CD80- or CD86-expressing T cells, B cells, NK cells, CD11b+ myeloid cells, and DCs were added and set at 100%; the relative contribution of individual immune cell subsets to overall CD80 or CD86 expression was then calculated accordingly, and error bars were omitted for clarity. (D) CD80 and CD86 expressions by naïve (CD44lo) and memory phenotype (CD44hi) T cell subsets. All data are standard errors of the means (SEM) determined for 3 mice/group.
Virus control and global CD8+ and CD4+ T cell immunity under conditions of CD80/86 deficiency.
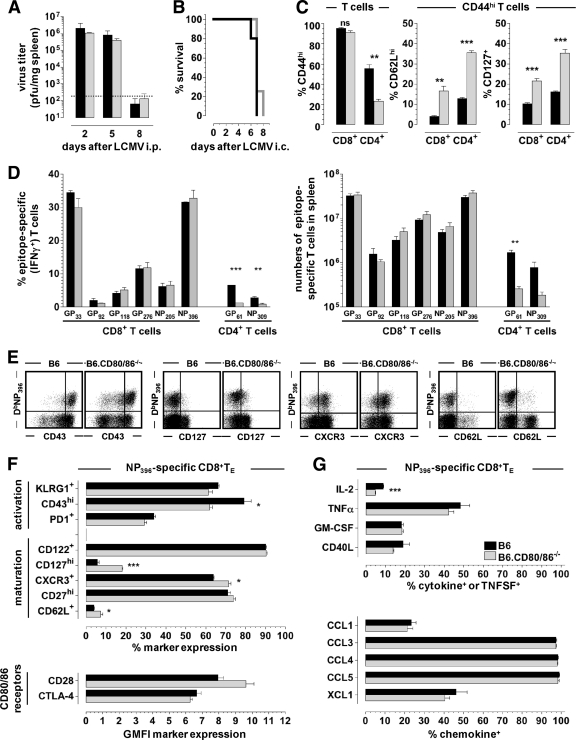
The LCMV model constitutes one of the most widely used experimental systems for the study of T cell-dependent virus control and immunopathology, yet to date, only a single recent investigation has evaluated LCMV-specific T cell immunity directly in B6.CD80/86−/− mice (28). Our initial evaluation of major parameters such as virus clearance kinetics after peripheral (i.p.) LCMV challenge and rapid death after intracranial infection demonstrated no alterations as a consequence of the CD80/86 deficiency (Fig. 2A and B) and therefore suggested the effective generation of LCMV-specific TE immunity. However, we also noted a specific decrease in numbers of activated (CD44hi) CD4+ T cells and, unexpectedly, elevated CD62L and CD127 expression levels by both CD44hi CD4+ and CD8+ T cells in B6.CD80/86−/− mice (Fig. 2C). A more detailed quantification of epitope-specific CD8+ and CD4+ TE populations confirmed the normal expansion of LCMV-specific CD8+ TE but a reduced antigen-driven proliferative expansion of CD4+ TE in the systemic absence of CD80/86 (Fig. 2D). Since the individual epitope-specific T cell populations differ not only according to immunodominance but also to activation threshold (39), our findings further indicate that CD80/86-mediated costimulatory interactions do not differentially modulate TE populations of disparate functional avidities.
Fig 2.
Virus control and effector T cell responses in the absence of CD80/86. (A) B6 mice (black) and B6.CD80/86−/− mice (gray) were infected with 2 × 105 PFU LCMV Armstrong (Arm) i.p., followed by determinations of viral titers in the spleen at the indicated time points. The dotted line indicates the detection threshold of 2 × 102 PFU/mg tissue. (B) Rapid death of both B6 (black) and B6.CD80/86−/− (gray) mice following i.c. infection with 103 PFU LCMV Arm. (C) Frequencies of CD44hi T cells (left) and CD62Lhi or CD127+ T cells within the CD44hi T cell compartments (right) of B6 mice (black) and B6.CD80/86−/− mice (gray) 8 days after LCMV Arm i.p. challenge. ns, not significant. (D) Frequencies (left) and absolute numbers (right) of epitope-specific CD8+ and CD4+ T cells in the spleen determined by intracellular IFN-γ staining. All bar diagrams display SEM (3 to 5 mice/group). (E) Phenotypes of NP396-specific CD8+ TE (plots are gated on CD8+ T cells on day 8 after LCMV challenge). (F) Summary of phenotypic analyses of DbNP396+ CD8+ TE (day 8) in B6 (black) and B6.CD80/86−/− (gray) mice. CTLA-4 expression levels refer to both surface and intracellular CTLA-4 levels, and no significant differences were recorded for CD49d, CD223, or CD244 expression (not shown). (G) Inducible cytokine, TNFSF, and chemokine production assessed by brief in vitro restimulation of CD8+ TE with the NP396 peptide as detailed in Materials and Methods. The bar diagrams display the fraction of NP396-specific (IFN-γ+) CD8+ TE producing the indicated additional cytokines, TNFSFs, or chemokines (representative data from 2 to 3 experiments are SEM for 3 to 4 mice/group).
Phenotypic and functional alterations of antiviral CD8+ TE responses in CD80/86-deficient mice.
To delineate potential phenotypic changes among CD80/86-deficient CD8+ TE, we selected a panel of cell surface receptors, the expressions of which are either downregulated (“activation markers”) or upregulated (“maturation markers”) in the transition from TE to TM stage and beyond (30, 34, 78, 79); we also included analyses of CD28 and CTLA-4, as their expression levels may be influenced by the systemic absence of CD80/86. Despite their apparently normal proliferative expansion, LCMV-specific CD8+ TE populations generated in B6.CD80/86−/− mice consistently exhibited several subtle phenotypic alterations, such as increased CD62L, CD127, and CXCR3 as well as reduced CD43 (activation-associated isoform) expression levels (Fig. 2E and F). While these patterns may reflect the transduction of “weaker” stimuli and thus may indicate either inefficient phenotypic CD8+ TE conversion (e.g., incomplete CD62L/CD127 downregulation) or accelerated CD8+ TM differentiation (e.g., earlier CD62L/CD127 reexpression), our results clearly demonstrate a partial uncoupling of proliferative expansion and the associated phenotypic modulation. Lastly, at the level of induced cytokine and TNFSF production, we noted only a slight reduction of the IL-2 synthesis capacity of CD80/86-deficient CD8+ TE, and a specific interrogation of chemokine production (19) revealed no differences between experimental and control CD8+ TE populations (Fig. 2G).
No role for CD8+ T cell-expressed CD80/86 in the acquisition of phenotypic and functional alterations.
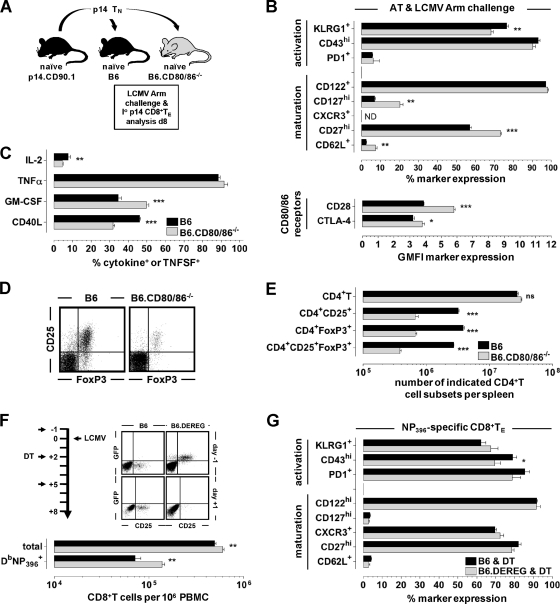
Given the high levels of CD86 expression by naïve CD8+ T cells (Fig. 1D), we addressed the possibility of altered phenotypic and functional CD8+ TE properties in CD80/86-deficient mice as a consequence of their own lack of CD80/86. To this extent, we employed the “p14 chimera” system, i.e., the adoptive transfer (AT) of naïve p14 cells (TCRtg CD8+ T cells specific for the LCMV-GP33-41 determinant) into B6 versus B6.CD80/86−/− hosts and subsequent LCMV challenge. As shown in Fig. 3A to C, p14 TE generated in CD80/86-deficient recipients acquired phenotypic and functional properties similar to those of endogenous CD8+ TE primed in B6.CD80/86−/− mice (Fig. 2E to G). Thus, the lack of CD80/86 expression by non-T cells, most likely APCs, imparts subtle alterations to the differentiation of virus-specific CD8+ TE populations regardless of their CD80/86 expression status.
Fig 3.
Role of T cell-expressed CD80/86 and TREGs in generation of primary CD8+ TE responses. (A) To generate p14 chimeras, 2 × 103 p14 TCRtg CD8+ T cells purified from naïve CD90.1+ p14 mice were adoptively transferred into congenic B6 or B6.CD80/86−/− mice that were subsequently infected with 2 × 105 PFU LCMV Arm i.p. (B and C) Phenotypic (B) and functional (C) analyses of splenic p14 TE were conducted 8 days later (data are SEM [n = 3]). ND, not determined; GMFI, geometric mean of fluorescence intensity. (D and E) Spleen cells obtained from naïve B6 and B6.CD80/86−/− mice were stained for CD4 and CD25 as well as intracellular FoxP3. The representative dot plots are gated on CD4+ cells (D), and the bar diagram (E) shows the absolute numbers of indicated CD4+ T cell subsets in the spleens of these mice (ns, not significant). Note the ∼7-fold reduction of CD4+ CD25+ FoxP3+ TREGs in naïve B6.CD80/86−/− mice (no differences in the level of FoxP3 mean fluorescence intensity [not shown]). (F) B6 and B6.DEREG mice were treated with diphtheria toxin (DT) on days −1, +2, and +5 (1.0 μg DT i.p.) in relation to LCMV infection. Dot plots are gated on CD4+ T cells analyzed right before the first DT injection (day −1) and 1 day after LCMV challenge (day +1). The bar diagram enumerates total and NP396-specific CD8+ T cells in the blood of DT-treated B6 (black) and B6.DEREG (gray) mice 8 days after infection. (G) Phenotypes of NP396-specific CD8+ TE analyzed on day 8 after challenge (peripheral blood mononuclear cells [PBMC]) (all data are SEM [n = 4 to 5]).
Role of TREGs in primary LCMV-specific CD8+ TE immunity.
In addition to the absence of CD80/86, CD80/86-deficient mice harbor reduced numbers of naturally occurring regulatory T cells (TREGs) (57, 68), and our own analyses of unmanipulated B6.CD80/86−/− mice demonstrated a specific ∼7-fold reduction of FoxP3+ CD25+ CD4+ TREGs in the presence of otherwise normal CD4+ T cell numbers (Fig. 3D and E). Interestingly, the relative dearth of TREGs in B6.CD80/86−/− mice can potentiate rather than curtail primary TE responses under certain experimental conditions, suggesting that CD80/86, principally via the maintenance of TREGs, can also exert inhibitory functions (52). If, however, TREGs contribute to the regulation of LCMV-specific T cell immunity at all is presently unknown, since the LCMV system has remained conspicuously absent from the catalog of viral pathogens, the T cell responses to which are restrained by TREG activity (66). To better define the role of TREGs in the context of a primary LCMV response, we used a model for inducible TREG ablation (46) and indeed observed up to a ∼2-fold increase in levels of LCMV-specific CD8+ TE populations albeit in the absence of major phenotypic alterations (Fig. 3F and G). It is therefore conceivable that the reduction in numbers of TREGs in B6.CD80/86−/− mice can partially compensate for potential impairments imparted by suboptimal CD8+ TE priming. In fact, a careful inspection of Fig. 2D shows that absolute numbers of LCMV-specific CD8+ TE in B6.CD80/86−/− mice tended to be slightly, though clearly not significantly, elevated.
Numerical, phenotypic, and functional alterations of primary LCMV-specific CD4+ TE populations under conditions of CD80/86 deficiency.
In contrast to CD8+ TE responses, the primary expansion of LCMV-specific CD4+ TE generated by CD80/86-deficient mice and quantified here by MHC class II (MHC-II) tetramer staining was profoundly diminished, and phenotypic alterations, including enhanced levels of CD62L and CD127 expression, were more pronounced than those in the CD8+ T cell compartment (Fig. 4A to C). Furthermore, we noted a broader range of impaired CD4+ TE functionalities, including a reduction of the induced chemokine production capacity (Fig. 4D). Importantly, however, the proliferative expansion of CD80/86−/− CD4+ TE was completely restored in wt hosts (Fig. 4E), indicating that CD80/86 expression by other cells, likely APCs, is sufficient to rescue primary CD4+ TE responses and allowing for the additional conclusion that B6.CD80/86−/− mice do not exhibit a reduction of naïve virus-specific precursors within their naïve CD4+ T cell pool. In summary, systemic CD80/86 deficiency curtails primary antiviral CD4+ TE immunity and imparts more subtle alterations to the differentiation of specific CD8+ TE but overall does not compromise effective virus clearance.
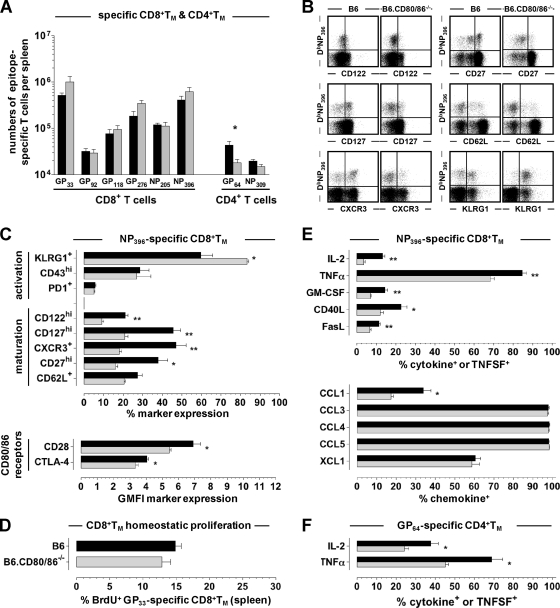
CD80/86 deficiency compromises the formation of CD8+ and CD4+ TM.
To assess the development of specific TM populations, we observed B6 and B6.CD80/86−/− mice for up to ∼3 months after acute LCMV Arm challenge. Infectious virus as determined by a plaque assay remained undetectable (spleen, liver, and kidney), and no viral mRNA was detected by qRT-PCR (59) (not shown). The frequencies and numbers of LCMV-specific CD8+ TM were not significantly different, although there appeared to be a trend toward enhanced CD8+ TM numbers in the B6.CD80/86−/− mice; specific CD4+ TM numbers, in contrast, but in agreement with their reduced primary expansion, were modestly reduced by a factor of up to ∼2.5 (Fig. 5A). The phenotypic appearance of specific CD8+ TM generated by B6.CD80/86−/− mice, however, demonstrated multiple alterations, such as increased KLRG1 as well as decreased CD27, CD62L, CD122, CD127, and CXCR3 expression levels (Fig. 5B and C). Collectively, these phenotypic changes, though not associated with altered CD8+ TM homeostasis (Fig. 5D), suggest a “stunted” CD8+ TM maturation process (34; data not shown) and potentially impaired secondary reactivity. Indeed, the spectrum of inducible effector functions elicited in CD80/86-deficient CD8+ TM was more limited, as evidenced by the impaired synthesis of IL-2, tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), CD40L, FasL, and CCL1 (Fig. 5E). Due to the low numbers of specific CD4+ TM present in LCMV-immune B6.CD80/86−/− mice, similar phenotypic and functional analyses did not consistently yield significant differences (not shown), yet we note that the impaired production of IL-2 and TNF-α was a reproducible feature of CD80/86-deficient CD4+ TM (Fig. 5F).
Fig 5.
Altered phenotypic and functional properties of LCMV-specific TM generated in B6.CD80/86-deficient mice. (A) Absolute numbers of epitope-specific CD8+ and CD4+ TM in the spleens of B6 (black) and B6.CD80/86−/− (gray) mice (84 days postinfection [dpi]) calculated after restimulation with different peptides and intracellular IFN-γ expression analysis. (B and C) Representative dot plots (gated on CD8+ T cells) and summary of DbNP396+ CD8+ TM phenotypes (54 dpi). No differences were observed for CD223 or CD244 expression (not shown). (D) Homeostatic proliferation of GP33-specific CD8+ TM determined after a 7-day BrdU pulse (77 to 84 dpi), as detailed in Materials and Methods. (E) Functional profiles of NP396-specific CD8+ TM analyzed at 54 dpi (cytokines and TNFSFs) or 62 dpi (chemokines). (F) Induced IL-2 and TNF-α production by GP64-specific (IFN-γ+) CD4+ TM determined at 54 dpi. All data are SEM for ≥3 mice/group and independent experiments performed 2 to 3 times.
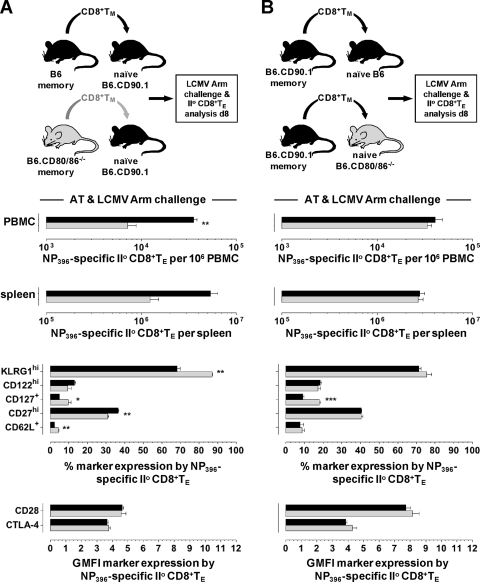
Impaired secondary TE immunity in the absence of CD80/86 and specific requirement of CD80 for efficient secondary CD8+ TE expansion.
For an evaluation of secondary TE immunity, we challenged LCMV-immune B6 and B6.CD80/86−/− mice with a high dose of the LCMV variant cl13, which causes a protracted/persistent infection in the absence of functional LCMV-specific T cell memory (11). Although immune B6.CD80/86−/− mice readily controlled cl13 infection, secondary expansions of specific CD8+ and CD4+ TE populations were curtailed by factors of ∼10 and ∼20, respectively, and were associated with functional impairments, notably reduced IL-2 production (Fig. 6A to C and data not shown). Compromised secondary immunity may result from the specific requirement of CD80/86-dependent costimulation in the context of secondary TE responses, defective TM development, or a combination thereof. To address the first possibility, we transferred CD8+ TM generated in congenic B6 mice into naïve B6 versus B6.CD80/86−/− hosts and quantified secondary CD8+ TE expansions in response to a cl13 challenge (Fig. 6D). Our results clearly support the notion that CD80/86-dependent costimulation is required for optimal secondary CD8+ TE expansion, yet the defect observed for B6.CD80/86−/− hosts, a ∼3-fold reduction compared to B6 recipients, was less pronounced than that observed for the rechallenged LCMV-immune B6.CD80/86−/− mice (Fig. 6B). Interestingly, the altered expressions of several cell surface antigens (KLRG1, CD62L, and CD127) by secondary CD8+ TE generated in B6.CD80/86−/− hosts phenocopied changes of primary CD8+ TE development in a similar setting (compare Fig. 6E to 3A and B), pointing toward common CD80/86-dependent differentiation patterns for CD8+ TE populations in general. Lastly, by use of individual CD80- and CD86-deficient recipients of B6 CD8+ TM, we found that the proliferative expansion of secondary CD8+ TE in response to an LCMV cl13 challenge was partially dependent on host-expressed CD80 but not CD86 (Fig. 6F).
Defective CD8+ TM differentiation in the absence of CD80/86 contributes to impaired secondary reactivity.
We next determined if the phenotypic and functional alterations of the CD8+ TM generated in B6.CD80/86−/− mice (Fig. 5B to E) would contribute to their compromised secondary reactivity. By use of a rechallenge protocol with LCMV Arm, secondary expansions of CD80/86-deficient CD8+ TE were decreased, and these cells presented with a “mixed” phenotype, as indicated by increased CD62L and CD127 levels (similar to primary CD8+ TE generated in a CD80/86−/− environment) but also elevated KLRG1 and reduced CD27 expression levels (similar to CD80/86−/− CD8+ TM) (Fig. 7A). We conclude that both defective CD8+ TM development under conditions of systemic CD80/86 deficiency and a specific requirement for CD80-dependent costimulation in the context of secondary CD8+ TE responses contribute to the profoundly impaired secondary reactivity observed for LCMV-immune B6.CD80/86−/− mice directly rechallenged with LCMV cl13 (Fig. 6B).
Fig 7.
CD80/86 deficiency and secondary CD8+ TE reactivity under conditions of LCMV Arm rechallenge. (A) CD8+ T cells were enriched from LCMV-immune B6 and B6.CD80/86−/− donors (62 to 64 dpi) by CD4+ T/B cell depletion, and populations containing 104 DbNP396+ CD8+ TM were transferred into congenic wt recipients, followed by LCMV Arm challenge (2 × 105 PFU i.p.) and analyses of secondary CD8+ TE expansions and phenotypes 8 days later (the phenotypes of concurrent primary DbNP396+ CD8+ TE generated by the CD90.1+ hosts were identical in recipients of B6 and B6.CD80/86−/− CD8+ TM [not shown]). (B) Experimental setup similar to that described above for panel A but using only LCMV-immune wt donors (193 dpi) and B6 versus B6.CD80/86−/− recipients. The same results were obtained in four independent experiments, including cotransfer experiments in which CD80/86−/− and congenic (CD90.1) wt CD8+ TM were monitored within the same congenic (CD45.1) hosts (not shown). II°, secondary.
Secondary CD8+ TE responses generated against LCMV Armstrong are CD80/86 independent but require CD28.
Additional experiments with the LCMV Arm system, however, i.e., after the transfer of congenic B6 CD8+ TM into B6 versus B6.CD80/86−/− hosts, consistently failed to demonstrate any defect in regard to secondary CD8+ TE expansions (Fig. 7B) and thus stand in contrast to very similar experiments using LCMV cl13 as the rechallenge virus (Fig. 6D) as well as the emerging consensus that secondary CD8+ TE responses against several viruses rely on productive CD28-CD80/86 costimulatory interactions (9). To address this conundrum, we determined secondary CD8+ TE expansions following the AT of wt CD8+ TM and LCMV Arm challenge under conditions of a systemic CD28 blockade and found them to be significantly compromised (Fig. 8A). The specific contribution of T cell-expressed CD28 was further interrogated by use of CD28-deficient mice. In contrast to CD80/86-deficient mice, the absence of CD28 was not associated with a phenotypic modulation of virus-specific CD8+ TM (Fig. 8B), and a direct comparison of secondary reactivities by the combined AT of congenic wt and CD28−/− CD8+ TM and subsequent LCMV Arm infection confirmed the requirement of CD8+ TM-expressed CD28 for optimal secondary CD8+ TE expansion (Fig. 8C). It should be noted that the impaired proliferative expansion of CD28−/− secondary CD8+ TE was also observed in experiments in which wt and CD28−/− CD8+ TM were transferred into separate recipients (not shown), thus ruling out a competitive disadvantage of CD28−/− T cells in the combined AT/rechallenge experiments described above.
An impaired accumulation of CD28-deficient secondary CD8+ TE populations may emerge as a result of reduced proliferation and/or enhanced cell death. Here, we employed the combined AT/rechallenge system shown in Fig. 8C and assessed the ex vivo viability (annexin V-propidium iodide [PI] staining) and proliferation of secondary CD8+ TE after a brief BrdU pulse (6 h) on days 6 and 8 after LCMV Arm challenge. Interestingly, while the compromised expansion of CD28−/− secondary CD8+ TE was associated with reduced BrdU incorporation, these cells also exhibited a small but not significant reduction of annexin V binding (Fig. 8D and not shown). A similar observation of enhanced proliferation and accumulation of CD8+ TE despite a slight reduction of their ex vivo viability was also reported previously for other model systems (77), and we conclude that the CD28-mediated signals regulate secondary CD8+ TE expansions primarily via an augmentation of their proliferative capacities. In summary, the optimal elaboration of secondary CD8+ TE responses in the LCMV Arm model requires CD8+ TM-expressed CD28 but surprisingly not host-expressed CD80/86. The most straightforward explanation for this unexpected phenomenon is the existence of a novel CD28 ligand in addition to CD80/86, and while investigations to this extent are currently ongoing, we note that a very recent report documented a new CD28 ligand, the B7 family member ICOSL/B7-H2, in humans but apparently not in mice (83).
CD8+ TM primed in the absence of TREGs generate more robust secondary CD8+ TE responses.
While the targeted interrogation of T cell phenotypes can provide important clues about altered TE and/or TM differentiation, their predictive value as to in vivo secondary CD8+ TE activity is more limited, as evidenced here by the divergent CD8+ TM phenotypes in B6.CD80/86−/− (altered phenotypes) (Fig. 5C) and B6.CD28−/− (normal phenotypes) (Fig. 8B) mice but the similar degree of impaired secondary expansion after AT into wt hosts and LCMV Arm challenge (Fig. 7A and 8C). This conclusion is further supported by relating the phenotypes of CD8+ TM generated under conditions of impaired costimulation to those of CD8+ TM that have undergone a slightly enhanced primary response as a consequence of TREG depletion (Fig. 8E). Curiously, the “mixed phenotype” of CD8+ TM in B6.DEREG mice most closely resembled that of secondary CD80/86-deficient CD8+ TE (compare Fig. 8E to 7A), but secondary expansions of B6.DEREG CD8+ TM populations were significantly increased after both LCMV Arm and cl13 challenges (Fig. 8F). Please note that the enhanced secondary reactivity of this CD8+ TM population is a T cell-intrinsic feature owing to the experimental design (AT of CD8+ TM into wt hosts) and LCMV rechallenge in the absence of TREG depletion. In fact, to the best of our knowledge, our TREG experiments constitute the first evidence that TREG activity restrains both secondary and primary CD8+ TE responses in the LCMV system.
DISCUSSION
As sketched out in the introduction, a more detailed understanding of the role of CD28-CD80/86 costimulation in the regulation of antiviral T cell immunity is contingent upon an integration of experimental findings obtained from multiple model systems as well as a careful evaluation of the nature, sensitivity, and precise context of the methodologies employed for analyses of virus-specific T cell immunity and the associated symptomatology. Based on our use of the LCMV system, a model traditionally regarded as “costimulation independent,” and the demonstration that the lack of CD80/86 compromises all stages of the LCMV-specific T cell response, we propose that CD80/86-mediated signals indeed operate as nonredundant costimuli in the regulation of antiviral T cell immunity at large. The extent to which defined T cell defects impart clinically relevant consequences, however, can be ascertained only within the context of specific model systems; conversely, an absence of relevant symptomatology observed under conditions of impaired CD28-CD80/86 costimulation can assist in the definition of “safety thresholds” for therapeutic interventions aimed at curtailing T cell immunity by costimulation blockade.
Our experimental results confirm multiple observations made for several distinct model systems, including a very recent report on LCMV-specific T cell immunity in CD80/86-deficient mice (28), and reveal novel details that collectively can serve to better define the constraints and limitations of CD80/86 costimulation in antiviral T cell immunity. For example, the direct comparison of LCMV-specific CD8+ and CD4+ TE phenotypes in wt and CD80/86−/− mice (Fig. 2E and F and 4B and C), not previously undertaken in any study investigating virus-specific T cell immunity in the context of impaired CD28-CD80/86 costimulation, demonstrates predominantly quantitative rather than qualitative differences; i.e., the extent of differential marker expression by CD80/86−/− T cells (e.g., increased CD62L and CD127) is more pronounced for CD4+ than for CD8+ TE. A similar enhancement of CD62L or CD127 expression was recently reported for primary VACV-, MCMV-, and PyV-specific CD8+ TE responses (generated in CD28−/− or CD80/86−/− mice or under conditions of combined CD80/86 and CD40L blockade, respectively [3, 41, 67]) as well as for secondary influenza virus-specific CD4+ TE responses analyzed after CTLA-4Ig treatment (74). Since the reported phenotypic changes were associated with reduced proliferative expansions of corresponding TE populations, our results now specify that the more pronounced CD62Lhi/CD127hi phenotype is in fact uncoupled from the relative magnitude of the TE response (compare normal CD8+ TE and reduced CD4+ TE burst sizes in CD80/86−/− mice in Fig. 2D). Furthermore, the same changes were observed for both primary and secondary wt CD8+ TE generation in CD80/86−/− hosts (Fig. 3B and 6E), thus demonstrating that these phenotypic alterations emerge as a consequence of suboptimal TE priming in a CD80/86-deficient environment regardless of the MHC restriction (both CD8+ and CD4+ T cells), relative proliferative expansion, and challenge context (both primary and secondary responses). Very similar considerations also apply to the impaired functionalities of CD8+ and CD4+ TE primed in the absence of CD80/86 (Fig. 2G, 3C, and 4D); in particular, our analyses of induced chemokine synthesis by in vivo-primed virus-specific TE, prompted by the previously reported inhibition of CCL3 secretion by in vitro-generated CD28−/− or anti-CD80/86 antibody-blocked CD4+ TE (33), showed an expanded array of functional defects that pertain to multiple chemokines produced by CD80/86−/− CD4+ TE (CCL1, CCL3, CCL4, and CCL5) (Fig. 4D). The specific implication of CD28-CD80/86 signaling in the regulation of CD4+ TE chemokine production profiles is further underscored by our observation that the lack of another major costimulatory molecule, CD40L, did not diminish chemokine synthesis by LCMV-specific CD4+ TE (not shown).
Elevated expression levels of CD62L and/or CD127 by virus-specific CD8+ TE have also been reported as a result of higher numbers of naïve p14 or OT-I TCRtg cells used for the generation of the respective p14 and OT-I chimeras and were associated with an accelerated/improved transition to the CD8+ TM stage (4, 56). While we indeed observed a marginal enhancement of specific CD8+ TM numbers in CD80/86−/− mice (a difference that was reported previously by Grujic et al. to be significant for LCMV Arm-immune CD80/86−/− mice [28]) as well as a ∼3-fold diminution of the relative differences between wt and CD80/86−/− CD4+ T cell numbers in the effector versus the memory stage (Fig. 2D, 4A, and 5A), any potentially improved recruitment of CD80/86-deficient TE into TM populations was accompanied by more pronounced phenotypic alterations and functional impairments (Fig. 5). We note, however, that defective TM differentiation in CD80/86-deficient mice was not associated with compromised virus control (determined by plaque assay and qRT-PCR) or altered homeostatic proliferation rates of specific CD8+ TM (Fig. 5D). The collective impairment of CD8+ TM “maturation” markers (CD27, CD62L, CD122, CD127, and CXCR3) and inducible functionality (IL-2, GM-CSF, TNF-α, CD40L, FasL, and CCL1), i.e., the failure of CD80/86−/− CD8+ TM to reexpress and progressively upregulate these receptors and to efficiently synthesize cytokines, TNFSFs, and chemokines upon in vitro restimulation, echoes and extends recent observations of altered CD80/86- and/or CD28-deficient CD8+ TM phenotypes/functionalities in models of both low-level virus persistence (MHV-68 and LCMV Traub) and effective virus clearance (VACV and LCMV Arm) (22, 24, 28). In our model system, the selective reduction of CCL1 synthesis among CD80/86−/− CD8+ TM-produced chemokines (Fig. 5E) is especially noteworthy, since we recently identified inducible CCL1 expression as a unique and distinctive marker for “polyfunctional” TM populations (J. Eberlein and D. Homann, unpublished observations). In summary, the defective differentiation of virus-specific CD8+ TM in the absence of functional CD28-CD80/86 costimulation, just as the original CD8+ TE generation preceding it, remains at least in part uncoupled from T cell response kinetics/magnitude and efficient virus control.
In regard to secondary TE activity, earlier work with viruses such as influenza virus and MHV-68 documented impaired CD8+ TM recall responses in the absence of functional CD28-CD80/86 interactions, but any interpretation of the experimental results was confounded by the lower levels of CD8+ TM present in virus-immune CD28−/− or CD80/86−/− mice (7, 8, 22, 32). More recent studies, however, have shown that CD28-CD80/86 costimulation is indeed required within the specific context of secondary virus-specific CD8+ TE immunity (10, 24, 25, 28), and our study provides further evidence in support of both concepts, namely, that CD28-CD80/86 costimulation is required for the development of fully functional CD8+ TM as well as for the elaboration of efficient recall responses. Using experimental protocols that comprise the direct rechallenge of LCMV Arm-immune CD80/86−/− mice that harbor normal if not elevated CD8+ TM numbers (Fig. 5A and 6B and C) and complementary AT settings (wt CD8+ TM into CD80/86−/− recipients [Fig. 6D and E] and CD80/86−/− CD8+ TM into wt recipients [Fig. 7A]), we found that (i) the generation of CD8+ TM in the absence of CD80/86 confers intrinsic functional defects that inhibit their capacity for secondary expansion, (ii) wt CD8+ TM require CD80/86 costimulation for the elaboration of secondary responses, and (iii) the above-described defects are compounded if CD80 and CD86 are absent during both primary and secondary challenges.
Unexpectedly, however, the precise costimulation requirement for wt CD8+ TM was restricted to host-expressed CD80 in the cl13 rechallenge system (Fig. 6F). Inasmuch as a potentially unique contribution of CD80 or CD86 to the regulation of antiviral T cell immunity has been investigated, mostly redundant functions have been reported, with two notable exceptions: CD86 operates as the dominant and exclusive functional CD28 ligand in the regulation of primary influenza virus- and VACV-specific CD8+ TE responses, respectively (53, 67). We have shown previously that direct LCMV infection of APCs in vivo enhances CD80 and CD86 expressions to an equivalent extent (38) such that the specific requirement of CD80 for secondary CD8+ TE populations responding to LCMV cl13 may be related to the more restricted CD80 versus CD86 expression patterns under steady-state conditions (Fig. 1), but we also note that the role of differential CD80 and CD86 functions remains a topic of lively speculation (27, 67). Even more surprising was our observation that, in contradistinction to the cl13 rechallenge experiments, both CD80 and CD86 were completely dispensable for wt CD8+ TM responses to an LCMV Arm rechallenge (Fig. 7B), yet secondary proliferation and expansion of CD8+ TE in this scenario nevertheless required CD28, as shown by AT experiments using CD28−/− CD8+ TM or wt CD8+ TM in conjunction with an anti-CD28 blockade (Fig. 8A to D). Thus, in addition to the possible existence of another CD80/86 receptor, as discussed above (and, in principle, compatible with the distinct LCMV-specific CD8+ TM phenotypes in CD80/86−/− versus CD28−/− mice reported previously by Grujic et al. [28] and also shown here [Fig. 5C versus 8B]), we have now obtained functional in vivo evidence that CD8+ TM may productively interact with a novel CD28 ligand. In humans, such a ligand was recently identified (ICOSL/B7-H2), and while CD28-ICOSL interactions appear not to take place mice (83), it is not unreasonable to consider the possibility of other murine CD28 ligands among known or novel B7 family members (16).
We further explored the potential role of two additional features that require consideration in studies conducted with CD80/86-deficient mice: the absence of T cell-expressed CD80/86 as well as the relative dearth of TREGs (27, 57, 68). The expression of CD80/86 by immune cells other than APCs has long been appreciated, but the degree of baseline CD80/86 expression, in particular the high levels of CD86 on naïve CD8+ T cells, is nonetheless remarkable (Fig. 1). If and under what conditions CD80 and CD86 may serve as inhibitory T cell receptors, for example, as targets for CTLA-4-bearing TREGs, remain a matter of ongoing debate (27, 62), and our studies indicate that T cell-expressed CD80/86 did not modulate LCMV-specific T cell immunity: phenotypic alterations and functional impairments of primary CD8+ TE resulted as a consequence of a CD80/86 deficiency by non-T cells irrespective of CD80/86 expression by responding CD8+ TE (Fig. 2F and G and 3A to C), and notably, primary responses of CD80/86−/− CD4+ TE were rescued but not further improved in CD80/86-sufficient hosts (Fig. 4E). The notion of inhibitory rather than stimulatory CD80/86 activities has also been extended to “reverse signaling,” indoleamine 2,3-dioxygenase (IDO) induction, TREGs, and their reliance on CD80/86 interactions (52, 64). While our previous work has documented that IDO contributes to only a modest inhibition of primary LCMV-specific CD4+ but not CD8+ TE responses (35), no reports that document the inhibition of LCMV-specific CD8+ TE immunity by TREGs have been published to date. The observation that TREG depletion can in fact enhance primary and, in particular, secondary LCMV-specific CD8+ TE responses (Fig. 3F and 8F) is therefore important, as it demonstrates a “conventional” role for TREGs even in the LCMV model, an experimental system previously considered “TREG independent” (66).
Finally, we draw attention to the more restricted utility of the phenotypic and functional aberrations recorded by us and others for CD8+ TM generated in the absence of functional CD28-CD80/86 interactions as it relates to the clinical parameters of morbidity and mortality. The effective immune protection observed for LCMV-immune CD80/86−/− mice after direct cl13 rechallenge (Fig. 6A) essentially reproduced the previously reported phenotype of CD28−/− mice (72) despite a substantial (10- to 20-fold) reduction of secondary CD80/86-deficient TE expansions and the accompanying functional impairments (Fig. 6B and C). Even when the degree of secondary proliferation by reactive CD8+ TE populations is used as the principal experimental readout, complementary AT experiments demonstrated similar defects for CD8+ TM with both altered (CD80/86−/− CD8+ TM) (Fig. 5C and 7A) and normal (CD28−/− CD8+ TM) (Fig. 8B and C) phenotypes and even enhanced secondary reactivity for CD8+ TM with a “mixed” phenotype (CD8+ TM generated in the absence of TREGs) (Fig. 8E and F). In fact, the studies reported to date that investigated virus control in AT settings to specifically interfere with CD28-CD80/86 costimulation only in the context of secondary infections reported only delayed but not an inhibited eradication of influenza virus, HSV-1, or LCMV (10, 25), and CTLA-4Ig blockade may even exert beneficial effects by limiting the immunopathology caused by secondary influenza virus-specific CD4+ TE (74). The precise role of CD28-CD80/86 costimulation clearly will require additional and refined investigations conducted with other viral pathogen systems, but current evidence suggests that antiviral secondary TE responses are sufficiently robust such that therapeutic interference with CD28-CD80/86 costimulation may be conducted within a certain “safety margin.”
ACKNOWLEDGMENTS
This work was supported by NIH grant AG026518-01, Juvenile Diabetes Research Foundation (JDRF) CDA 2-2007-240, a BDC Pilot and Feasibility (P&F) grant (D.H.), and Diabetes and Endocrinology Research Center (DERC) grant P30-DK057516.
We thank J. Kurche and R. Kedl (National Jewish Health) for advice about DT treatment of B6.DEREG mice, R. Wong (UCD/BDC Molecular Core facility) for assistance with LCMV-GP qRT-PCR, and the NIH tetramer core facility for the provision of MHC-peptide complexes.
Footnotes
Published ahead of print 7 December 2011
REFERENCES
- 1. Andreasen SO, Christensen JE, Marker O, Thomsen AR. 2000. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J. Immunol. 164:3689–3697 [DOI] [PubMed] [Google Scholar]
- 2. Arens R, et al. 2011. B7-mediated costimulation of CD4 T cells constrains cytomegalovirus persistence. J. Virol. 85:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arens R, et al. 2011. Differential B7-CD28 costimulatory requirements for stable and inflationary mouse cytomegalovirus-specific memory CD8 T cell populations. J. Immunol. 186:3874–3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badovinac VP, Haring JS, Harty JT. 2007. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity 26:827–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertram EM, et al. 2004. A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J. Immunol. 172:981–988 [DOI] [PubMed] [Google Scholar]
- 6. Bertram EM, Dawicki W, Watts TH. 2004. Role of T cell costimulation in anti-viral immunity. Semin. Immunol. 16:185–196 [DOI] [PubMed] [Google Scholar]
- 7. Bertram EM, Lau P, Watts TH. 2002. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 168:3777–3785 [DOI] [PubMed] [Google Scholar]
- 8. Bertram EM, et al. 2002. Role of ICOS versus CD28 in antiviral immunity. Eur. J. Immunol. 32:3376–3385 [DOI] [PubMed] [Google Scholar]
- 9. Boesteanu AC, Katsikis PD. 2009. Memory T cells need CD28 costimulation to remember. Semin. Immunol. 21:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borowski AB, et al. 2007. Memory CD8+ T cells require CD28 costimulation. J. Immunol. 179:6494–6503 [DOI] [PubMed] [Google Scholar]
- 11. Borrow P, Oldstone MBA. 1997. Lymphocytic choriomeningitis virus, p 593–627 In Nathanson N., et al. (ed), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- 12. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. 2007. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butte MJ, Pena-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. 2008. Interaction of human PD-L1 and B7-1. Mol. Immunol. 45:3567–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen W, Bennink JR, Morton PA, Yewdell JW. 2002. Mice deficient in perforin, CD4+ T cells, or CD28-mediated signaling maintain the typical immunodominance hierarchies of CD8+ T-cell responses to influenza virus. J. Virol. 76:10332–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christensen JE, et al. 2002. Role of CD28 co-stimulation in generation and maintenance of virus-specific T cells. Int. Immunol. 14:701–711 [DOI] [PubMed] [Google Scholar]
- 16. Compugen 2011. Compugen announces initial validation of additional B7/CD28 protein family based product candidate. Press release. Compugen, Tel Aviv, Israel: http://www.cgen.com/Content.aspx?Page=press_releases&NewsId=554 [Google Scholar]
- 17. Cook CH, et al. 2009. CD28/B7-mediated co-stimulation is critical for early control of murine cytomegalovirus infection. Viral Immunol. 22:91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dias P, et al. 2010. CD4 T-cell help programs a change in CD8 T-cell function enabling effective long-term control of murine gammaherpesvirus 68: role of PD-1-PD-L1 interactions. J. Virol. 84:8241–8249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eberlein J, et al. 2010. Comprehensive assessment of chemokine expression profiles by flow cytometry. J. Clin. Invest. 120:907–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edelmann KH, Wilson CB. 2001. Role of CD28/CD80-86 and CD40/CD154 costimulatory interactions in host defense to primary herpes simplex virus infection. J. Virol. 75:612–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fang M, Sigal LJ. 2006. Direct CD28 costimulation is required for CD8+ T cell-mediated resistance to an acute viral disease in a natural host. J. Immunol. 177:8027–8036 [DOI] [PubMed] [Google Scholar]
- 22. Fuse S, et al. 2006. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J. Virol. 80:9159–9170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fuse S, Tsai CY, Rommereim LM, Zhang W, Usherwood EJ. 2010. Differential requirements for CD80/86-CD28 costimulation in primary and memory CD4 T cell responses to vaccinia virus. Cell. Immunol. 266:130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fuse S, Zhang W, Usherwood EJ. 2008. Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J. Immunol. 180:1148–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garidou L, Heydari S, Truong P, Brooks DG, McGavern DB. 2009. Therapeutic memory T cells require costimulation for effective clearance of a persistent viral infection. J. Virol. 83:8905–8915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gett AV, Hodgkin PD. 2000. A cellular calculus for signal integration by T cells. Nat. Immunol. 1:239–244 [DOI] [PubMed] [Google Scholar]
- 27. Greenwald RJ, Freeman GJ, Sharpe AH. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515–548 [DOI] [PubMed] [Google Scholar]
- 28. Grujic M, et al. 2010. The role of CD80/CD86 in generation and maintenance of functional virus-specific CD8+ T cells in mice infected with lymphocytic choriomeningitis virus. J. Immunol. 185:1730–1743 [DOI] [PubMed] [Google Scholar]
- 29. Halstead ES, Mueller YM, Altman JD, Katsikis PD. 2002. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat. Immunol. 3:536–541 [DOI] [PubMed] [Google Scholar]
- 30. Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. 2000. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J. Exp. Med. 191:1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hashiguchi M, et al. 2008. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc. Natl. Acad. Sci. U. S. A. 105:10495–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hendriks J, Xiao Y, Borst J. 2003. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med. 198:1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herold KC, et al. 1997. Regulation of C-C chemokine production by murine T cells by CD28/B7 costimulation. J. Immunol. 159:4150–4153 [PubMed] [Google Scholar]
- 34. Hikono H, et al. 2007. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 204:1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Homann D, et al. 2006. Lack of intrinsic CTLA-4 expression has minimal effect on regulation of antiviral T-cell immunity. J. Virol. 80:270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Homann D, et al. 2002. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity 16:403–415 [DOI] [PubMed] [Google Scholar]
- 37. Homann D, et al. 2007. Mapping and restriction of a dominant viral CD4+ T cell core epitope by both MHC class I and MHC class II. Virology 363:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Homann D, McGavern DB, Oldstone MB. 2004. Visualizing the viral burden: phenotypic and functional alterations of T cells and APCs during persistent infection. J. Immunol. 172:6239–6250 [DOI] [PubMed] [Google Scholar]
- 39. Homann D, Teyton L, Oldstone MB. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913–919 [DOI] [PubMed] [Google Scholar]
- 40. Homann D, et al. 1998. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J. Virol. 72:9208–9216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kemball CC, et al. 2006. Costimulation requirements for antiviral CD8+ T cells differ for acute and persistent phases of polyoma virus infection. J. Immunol. 176:1814–1824 [DOI] [PubMed] [Google Scholar]
- 42. Kim IJ, Flano E, Woodland DL, Blackman MA. 2002. Antibody-mediated control of persistent gamma-herpesvirus infection. J. Immunol. 168:3958–3964 [DOI] [PubMed] [Google Scholar]
- 43. Kim SK, Schluns KS, Lefrancois L. 1999. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 163:4125–4132 [PubMed] [Google Scholar]
- 44. Kopf M, et al. 2000. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 192:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kündig TM, et al. 1996. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity 5:41–52 [DOI] [PubMed] [Google Scholar]
- 46. Lahl K, et al. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee BJ, Reiter SK, Anderson M, Sarawar SR. 2002. CD28(−/−) mice show defects in cellular and humoral immunity but are able to control infection with murine gammaherpesvirus 68. J. Virol. 76:3049–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leitner J, et al. 2009. B7-H3 is a potent inhibitor of human T-cell activation: no evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 39:1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lenz DC, et al. 2004. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc. Natl. Acad. Sci. U. S. A. 101:9357–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Y. 1997. Is CTLA-4 a negative regulator for T-cell activation? Immunol. Today 18:569–572 [DOI] [PubMed] [Google Scholar]
- 51. Liu Y, Wenger RH, Zhao M, Nielsen PJ. 1997. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J. Exp. Med. 185:251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lohr J, Knoechel B, Jiang S, Sharpe AH, Abbas AK. 2003. The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat. Immunol. 4:664–669 [DOI] [PubMed] [Google Scholar]
- 53. Lumsden JM, Roberts JM, Harris NL, Peach RJ, Ronchese F. 2000. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J. Immunol. 164:79–85 [DOI] [PubMed] [Google Scholar]
- 54. Lyon AB, Sarawar SR. 2006. Differential requirement for CD28 and CD80/86 pathways of costimulation in the long-term control of murine gammaherpesvirus-68. Virology 356:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mandelbrot DA, et al. 2001. B7-dependent T-cell costimulation in mice lacking CD28 and CTLA4. J. Clin. Invest. 107:881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marzo AL, et al. 2005. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 6:793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. May KF, Jr, et al. 2007. B7-deficient autoreactive T cells are highly susceptible to suppression by CD4(+)CD25(+) regulatory T cells. J. Immunol. 178:1542–1552 [DOI] [PubMed] [Google Scholar]
- 58. McAdam AJ, Farkash EA, Gewurz BE, Sharpe AH. 2000. B7 costimulation is critical for antibody class switching and CD8(+) cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74:203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCausland MM, Crotty S. 2008. Quantitative PCR technique for detecting lymphocytic choriomeningitis virus in vivo. J. Virol. Methods 147:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McGavern DB, Truong P. 2004. Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells. J. Immunol. 173:4779–4790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reference deleted.
- 62. Paust S, Cantor H. 2005. Regulatory T cells and autoimmune disease. Immunol. Rev. 204:195–207 [DOI] [PubMed] [Google Scholar]
- 63. Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342:559–561 [DOI] [PubMed] [Google Scholar]
- 64. Puccetti P, Grohmann U. 2007. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat. Rev. Immunol. 7:817–823 [DOI] [PubMed] [Google Scholar]
- 65. Raue HP, Slifka MK. 2007. Pivotal advance: CTLA-4+ T cells exhibit normal antiviral functions during acute viral infection. J. Leukoc. Biol. 81:1165–1175 [DOI] [PubMed] [Google Scholar]
- 66. Rouse BT, Sarangi PP, Suvas S. 2006. Regulatory T cells in virus infections. Immunol. Rev. 212:272–286 [DOI] [PubMed] [Google Scholar]
- 67. Salek-Ardakani S, et al. 2009. Preferential use of B7.2 and not B7.1 in priming of vaccinia virus-specific CD8 T cells. J. Immunol. 182:2909–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Salomon B, et al. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12:431–440 [DOI] [PubMed] [Google Scholar]
- 69. Shahinian A, et al. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261:609–612 [DOI] [PubMed] [Google Scholar]
- 70. Sigal LJ, Reiser H, Rock KL. 1998. The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo. J. Immunol. 161:2740–2745 [PubMed] [Google Scholar]
- 71. Sprent J, Surh CD. 2002. T cell memory. Annu. Rev. Immunol. 20:551–579 [DOI] [PubMed] [Google Scholar]
- 72. Suresh M, et al. 2001. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J. Immunol. 167:5565–5573 [DOI] [PubMed] [Google Scholar]
- 73. Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 1999. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J. Immunol. 163:4859–4868 [PubMed] [Google Scholar]
- 74. Teijaro JR, et al. 2009. Costimulation modulation uncouples protection from immunopathology in memory T cell responses to influenza virus. J. Immunol. 182:6834–6843 [DOI] [PubMed] [Google Scholar]
- 75. Thebeau LG, Morrison LA. 2002. B7 costimulation plays an important role in protection from herpes simplex virus type 2-mediated pathology. J. Virol. 76:2563–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thebeau LG, Morrison LA. 2003. Mechanism of reduced T-cell effector functions and class-switched antibody responses to herpes simplex virus type 2 in the absence of B7 costimulation. J. Virol. 77:2426–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. West EE, et al. 2011. Tight regulation of memory CD8(+) T cells limits their effectiveness during sustained high viral load. Immunity 35:285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wherry EJ, et al. 2007. Molecular signature of CD8(+) T cell exhaustion during chronic viral infection. Immunity 27:670–684 [DOI] [PubMed] [Google Scholar]
- 79. Wherry EJ, et al. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234 [DOI] [PubMed] [Google Scholar]
- 80. Whitmire JK, Ahmed R. 2000. Costimulation in antiviral immunity: differential requirements for CD4(+) and CD8(+) T cell responses. Curr. Opin. Immunol. 12:448–455 [DOI] [PubMed] [Google Scholar]
- 81. Williams MA, et al. 2002. Persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J. Immunol. 169:5387–5391 [DOI] [PubMed] [Google Scholar]
- 82. Yamada A, et al. 2001. CD28-independent costimulation of T cells in alloimmune responses. J. Immunol. 167:140–146 [DOI] [PubMed] [Google Scholar]
- 83. Yao S, et al. 2011. B7-h2 is a costimulatory ligand for CD28 in human. Immunity 34:729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhu Y, Yao S, Chen L. 2011. Cell surface signaling molecules in the control of immune responses: a tide model. Immunity 34:466–478 [DOI] [PMC free article] [PubMed] [Google Scholar]