ResearchIn-Press PreviewGeneticsMuscle biology
Open Access |  10.1172/jci.insight.197759
10.1172/jci.insight.197759
Proteomics-based evaluation of AAV dystrophin gene therapy outcomes in mdx skeletal muscle
Erynn E. Johnson,1 Theodore R. Reyes,2 Jeffrey S. Chamberlain,2 James M. Ervasti,1 and Hichem Tasfaout2
1Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota - Twin Cities, Minneapolis, United States of America
2Department of Neurology, University of Washington School of Medicine, Seattle, United States of America
Find articles by Johnson, E. in: PubMed | Google Scholar
1Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota - Twin Cities, Minneapolis, United States of America
2Department of Neurology, University of Washington School of Medicine, Seattle, United States of America
Find articles by Reyes, T. in: PubMed | Google Scholar
1Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota - Twin Cities, Minneapolis, United States of America
2Department of Neurology, University of Washington School of Medicine, Seattle, United States of America
Find articles by
Chamberlain, J.
in:
PubMed
|
Google Scholar
|

1Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota - Twin Cities, Minneapolis, United States of America
2Department of Neurology, University of Washington School of Medicine, Seattle, United States of America
Find articles by Ervasti, J. in: PubMed | Google Scholar
1Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota - Twin Cities, Minneapolis, United States of America
2Department of Neurology, University of Washington School of Medicine, Seattle, United States of America
Find articles by Tasfaout, H. in: PubMed | Google Scholar
Published November 27, 2025 - More info
Copyright © 2025, Johnson et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
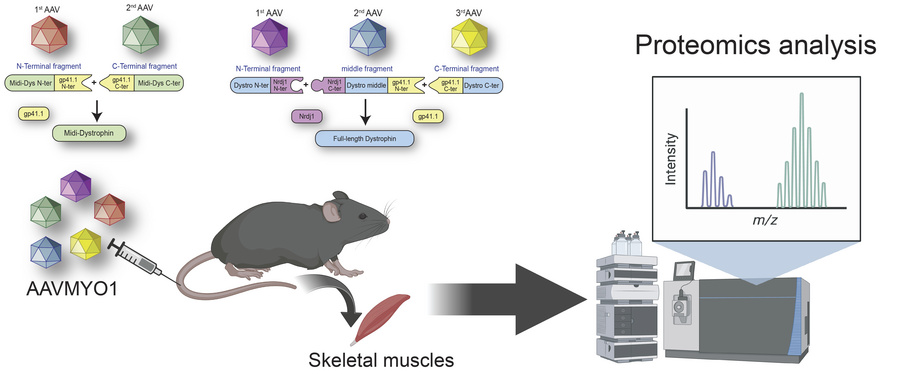
Duchenne muscular dystrophy (DMD) is a fatal genetic muscle-wasting disease characterized by loss of dystrophin protein. Therapeutic attempts to restore a functional copy of dystrophin to striated muscle are under active development, and many utilize adeno-associated viral (AAV) vectors. However, the limited cargo capacity of AAVs precludes delivery of full-length dystrophin, a 427 kDa protein, to target tissues. Recently, we developed a novel method to express large dystrophin constructs using the protein trans-splicing (PTS) mechanism mediated by split inteins and myotropic AAV vectors. The efficacy of this approach to restore muscle function in mdx4cv mice was previously assessed using histology, dystrophin immunolabeling, and western blotting. Here, we expand our molecular characterization of dystrophin constructs with variable lengths using a mass spectrometry-based proteomics approach, providing insight into unique protein expression profiles in skeletal muscles of wild-type, dystrophic mdx4cv, and AAV-treated mdx4cv. Our data reveal several affected cellular processes in mdx4cv skeletal muscles with changes in the expression profiles of key proteins to muscle homeostasis, whereas successful expression of dystrophin constructs results in an intermediate to complete restoration. This study highlights several biomarkers that could be used in future preclinical or clinical studies to evaluate the effectiveness of therapeutic strategies.
-
Version history
- Version 1 (November 27, 2025): In-Press Preview